Your new post is loading...

|
Scooped by
Juan Lama
|
The pharma, once a frontrunner in the race to develop the first vaccine for the respiratory virus, said it will end a 23,000-person study of its experimental shot amid a restructuring of its infectious disease division. Dive Brief: - Johnson & Johnson will stop developing its experimental vaccine for respiratory syncytial virus in an unexpected retreat from a high-profile research effort that had put the pharmaceutical giant among the leading companies seeking to win the first approval of a preventive shot.
- The company said Wednesday it will discontinue a 23,000-person Phase 3 trial, called Evergreen, of its RSV vaccine in adults following a review of its drug pipeline. The company does not plan to develop the shot for pregnant women or infants, a spokesperson confirmed.
- J&J’s pullback comes amid a restructuring of its infectious disease division, which was reported by Fierce Pharma in February. Its decision also thins the RSV vaccine competition, leaving GSK and Pfizer in the lead with shots that are currently under review by the Food and Drug Administration. Moderna is also developing an RSV vaccine and could file for approval this year.
Dive Insight: J&J’s decision to halt work on its RSV vaccine extends a series of setbacks for the company in infectious disease. In January, the company halted a large trial of an HIV vaccine after disappointing results, and last month, a partner company said J&J was dialing back its hepatitis B research amid the unit’s restructuring. The company successfully developed an effective vaccine for COVID-19, but concerns over rare side effects limited its use in the U.S., where it became a distant third choice after vaccines from Pfizer and Moderna. “The decision to discontinue the Company’s RSV adult vaccine program is part of a broader effort to make strategic choices for its pipeline and research and development investments to focus on medicines with the greatest potential benefit to patients,” J&J said in its statement Wednesday. J&J began the Evergreen trial in 2021 after data from a mid-stage study showed vaccination could prevent complications from RSV infection in older adults. The Phase 3 trial, which spanned more than 300 sites across 15 countries, made J&J one of the frontrunners in development of an RSV vaccine. No results have been made public from the study, but J&J would have had a high bar to clear the marks set by RSV vaccines from GSK and Pfizer. GSK’s shot, called Arexvy, proved 83% effective in preventing cases of lower respiratory infection caused by RSV infection when compared to a placebo in a Phase 3 study of older adults. Pfizer’s was 67% effective against moderate disease in that company’s late-stage trial. Both vaccines recently won the backing of an FDA panel, making approvals by the FDA later this spring more likely. (FDA advisers did express some concern around safety, particularly for Pfizer’s.) J&J will share data from the Evergreen study with the scientific community in the future, the spokesperson said. RSV infections are usually mild in healthy adults. But the disease can be more deadly in infants and older adults. Between 6,000 to 10,000 deaths occur from RSV in older adults each year in the U.S., according to data cited by the Centers for Disease Control and Prevention. Like other pharmas, J&J has shifted its focus more heavily to cancer and immune disease drugs, which it expects will help it reach a target of $60 billion in pharmaceutical sales by 2025. While J&J’s infectious disease unit sells several HIV treatments, it earns relatively little from vaccines. The company’s COVID vaccine generated sales of $120 million in the U.S. and $2 billion internationally last year. But in the fourth quarter, J&J reported no U.S. sales and incurred $821 million in costs related to scaling back its manufacturing of the shot. Wednesday’s announcement comes four years after another RSV setback for J&J. The pharma took a $900 million write-off for an RSV drug it had obtained as part of a $1.75 billion purchase of Alios BioPharma in 2014. The accounting charge followed the company’s decision to halt mid-stage clinical work on the drug. Ned Pagliarulo contributed reporting. Editor’s note: This story has been updated with additional detail throughout.

|
Scooped by
Juan Lama
|
BACKGROUND The Ad26.COV2.S vaccine was highly effective against severe–critical coronavirus disease 2019 (Covid-19), hospitalization, and death in the primary phase 3 efficacy analysis. METHODS We conducted the final analysis in the double-blind phase of our multinational, randomized, placebo-controlled trial, in which adults were assigned in a 1:1 ratio to receive single-dose Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe–critical Covid-19 with onset at least 14 days after administration and at least 28 days after administration in the per-protocol population. Safety and key secondary and exploratory end points were also assessed. RESULTS Median follow-up in this analysis was 4 months; 8940 participants had at least 6 months of follow-up. In the per-protocol population (39,185 participants), vaccine efficacy against moderate to severe–critical Covid-19 at least 14 days after administration was 56.3% (95% confidence interval [CI], 51.3 to 60.8; 484 cases in the vaccine group vs. 1067 in the placebo group); at least 28 days after administration, vaccine efficacy was 52.9% (95% CI, 47.1 to 58.1; 433 cases in the vaccine group vs. 883 in the placebo group). Efficacy in the United States, primarily against the reference strain (B.1.D614G) and the B.1.1.7 (alpha) variant, was 69.7% (95% CI, 60.7 to 76.9); efficacy was reduced elsewhere against the P.1 (gamma), C.37 (lambda), and B.1.621 (mu) variants. Efficacy was 74.6% (95% CI, 64.7 to 82.1) against severe–critical Covid-19 (with only 4 severe–critical cases caused by the B.1.617.2 [delta] variant), 75.6% (95% CI, 54.3 to 88.0) against Covid-19 leading to medical intervention (including hospitalization), and 82.8% (95% CI, 40.5 to 96.8) against Covid-19–related death, with protection lasting 6 months or longer. Efficacy against any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was 41.7% (95% CI, 36.3 to 46.7). Ad26.COV2.S was associated with mainly mild-to-moderate adverse events, and no new safety concerns were identified. CONCLUSIONS A single dose of Ad26.COV2.S provided 52.9% protection against moderate to severe–critical Covid-19. Protection varied according to variant; higher protection was observed against severe Covid-19, medical intervention, and death than against other end points and lasted for 6 months or longer. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722. opens in new tab.) Published in NEJM (Feb. 9, 2022): https://doi.org/10.1056/NEJMoa2117608

|
Scooped by
Juan Lama
|
A two-dose version of Johnson & Johnson's coronavirus vaccine provides 94% protection against symptomatic infection, the company said Tuesday -- making a two-dose regimen of J&J's Janssen vaccine comparable to a two-dose regimen of Moderna's or Pfizer's. Plus, the company said, adding a booster dose to a single shot of the vaccine raised immunity even more, and should also protect people strongly against infection. The company released some details of three studies looking at various aspects of its Janssen vaccine, and said that, taken together, they showed the vaccine provided long-lasting protection that could be boosted with an extra shot. "Our large real-world-evidence and Phase 3 studies confirm that the single-shot Johnson & Johnson vaccine provides strong and long-lasting protection against COVID-19-related hospitalizations," Dr. Mathai Mammen, global head of Janssen Research & Development, said in a statement. "Our single-shot vaccine generates strong immune responses and long-lasting immune memory. And, when a booster of the Johnson & Johnson COVID-19 vaccine is given, the strength of protection against COVID-19 further increases." Johnson & Johnson's single-dose vaccine was given emergency use authorization by the US Food and Drug Administration on February 27. It has been given to about 14.8 million Americans, according to the US Centers for Disease Control and Prevention. The company's ongoing Phase 2 trial of a two-dose regimen showed giving two doses 56 days apart provided 100% protection against severe Covid-19 and 94% protection against moderate to severe Covid-19 in the United States. Globally, the two-dose regimen provided 75% protection against moderate-to-severe Covid-19, the company said. A second study showed people given a booster shot six months or longer after their first dose had a 12-fold increase in antibodies -- compared to a four-fold increase for people who got a second dose at two months. So protection should be stronger if people get boosters later, Dr. Dan Barouch, head of Beth Israel Deaconess' Center for Virology and Vaccine Research, told CNN. "If you wait longer and have boost at six months or later then you likely will have better boost," said Barouch. Third, the company said a real-world evidence study of 390,000 people in the US, using health insurance records through July -- so covering the Delta variant -- showed the one-shot J&J vaccine was 81% effective at preventing hospitalizations. "The Johnson & Johnson single-shot COVID-19 vaccine showed vaccine effectiveness against COVID-19-related hospitalizations at 86% for participants younger than 60 years, and 78% for those 60 years and older," the company said. "Among 390,517 vaccinated and 1,524,153 matched unvaccinated individuals, vaccine effectiveness 79% for COVID-19 and 81% for COVID-19-related hospitalizations," the Janssen-led research team wrote in a study posted online in a preprint. "In high-Delta-incidence states, rates of observed COVID-19 were higher in both groups than in the national cohort," they added. "In these states, vaccine effectiveness for observed COVID-19 was 79% overall and 78% during June and July, the months where Delta variant incidence was highest," they added. Barouch, who has worked with Janssen to test the vaccine but who was not directly involved in the three studies, said people who got the Johnson & Johnson vaccine should be reassured by the data. "All the vaccines in the US have shown robust and durable protection against severe disease and hospitalization," he said. "Ultimately, the job of a vaccine is to keep you from being sick and keep you from going into the hospital and to keep you alive, and all of the vaccines are doing that." Data on the J&J vaccine has come later than data about the Moderna and Pfizer/BioNTech vaccines because J&J's was authorized around two months later. Johnson & Johnson has said it will submit all of this data to the FDA for potential consideration for adding a booster dose, and perhaps for consideration to authorize a two-dose regimen. The Janssen vaccine is made using a different technology from Moderna's and Pfizer's vaccines. They deliver messenger RNA or mRNA directly to the body wrapped in compounds called lipids. The J&J vaccine is made using an adenovirus, a common cold virus, that's been engineered so it can get into cells, but then stops. It delivers genetic instructions that way. Barouch said there is room for a variety of approaches. "A single shot gives robust and durable protection over a substantial period of time of time with minimal evidence of decline," Barouch said. "I think the single dose vaccine is a reasonable option for people and for countries that want a simple and convenient vaccine that can be administered quickly," he added. "For outstanding protection, then a second shot can be given at any time between two months and eight months -- and the longer you wait, the better." That, he said, is because the body mounts a variety of immune responses. Antibodies -- immune system proteins that can either flag an invader or directly attack and neutralize it -- build up quickly but can wane over time. The body also produces cells called B cells and T cells, and these contribute to longer-term protection. Stimulating B cells with a boost after time -- after they have become less active -- appears to cause them to generate fresh antibodies more effectively, he said. Barouch said the J&J vaccine may appear less effective in countries outside the United States because it was tested in many countries when variants were circulating that can evade the protection offered by vaccines. The Beta or B.1.351 variant is an example -- it has so-called escape mutations that help it hide from the immune response. It circulated widely in South Africa but has been outcompeted in the US by Delta, which does not appear to escape immune protection as well. Johnson & Johnson Press Release (Sept. 21, 2021):

|
Scooped by
Juan Lama
|
With concerns surrounding the Delta coronavirus variant rising globally, how effective are the current vaccines in the U.S. at protecting against the new version of the virus? According to medical experts, the three vaccines currently available each offer protection. Here’s a breakdown of each vaccine and what you should know: How effective are the COVID vaccines overall? In clinical trials, Moderna’s In clinical trials, Moderna's vaccine reported 94.1% effectiveness at preventing COVID-19 in people who received both doses. The Pfizer-BioNTech vaccine was said to be 95% effective. A new CDC study reported that a single dose of Pfizer's or Moderna's COVID vaccine was 80% effective in preventing infections. That number jumped to 90% two weeks after the second dose, the study on vaccinated health care workers showed. "These findings indicate that authorized mRNA COVID-19 vaccines are effective for preventing SARS-CoV-2 infection, regardless of symptom status, among working-age adults in real-world conditions," the U.S. agency wrote in the study. "COVID-19 vaccination is recommended for all eligible persons." Pfizer's vaccine, the only one currently authorized for use in children as young as 12, also showed heightened effectiveness among adolescents. Pfizer in late March released preliminary results from a vaccine study of 2,260 U.S. volunteers ages 12 to 15, showing there were no cases of COVID-19 among fully vaccinated adolescents compared with 18 among those given dummy shots. More intriguing, researchers found the kids developed higher levels of virus-fighting antibodies than earlier studies measured in young adults. The FDA said J&J’s vaccine offers strong protection against what matters most: serious illness, hospitalizations and death. One dose was 85% protective against the most severe COVID-19 illness, in a massive study that spanned three continents — protection that remained strong even in countries such as South Africa, where the variants of most concern were spreading at the time. The CDC reports J&J/Janssen vaccine was 66.3% effective in clinical trials at preventing COVID-19 illness in people who had no evidence of prior infection 2 weeks after receiving the vaccine. "The vaccine had high efficacy at preventing hospitalization and death in people who did get sick," the CDC notes. "No one who got COVID-19 at least four weeks after receiving the J&J/Janssen vaccine had to be hospitalized." It is not known if any of the three vaccines prevent the spread of the virus by people who are asymptomatic, though the CDC noted that "early evidence suggests that the J&J/Janssen vaccine might provide protection against asymptomatic infection." How effective are the vaccines against the new Delta variant? Data surrounding vaccine effectiveness with the Delta variant is so far limited. While studies have shown that the available vaccines work against variants, including the Delta variant, all two-dose vaccines offer significantly more protection following their second dose. Researchers in England studied how effective the two-dose AstraZeneca and Pfizer-BioNTech vaccines were against it, compared with the Alpha variant that was first detected in the U.K. The vaccines were protective for those who got both doses but were less so among those who got one dose. One recent study showed the Pfizer vaccine was 84% effective against the variant after two doses, but only 34% effective after the first dose. Moderna also announced Tuesday that a new study showed its vaccine also produced promising protection in a lab setting against the Delta variant and others currently circulating. “As we seek to defeat the pandemic, it is imperative that we are proactive as the virus evolves. We remain committed to studying emerging variants, generating data and sharing it as it becomes available. These new data are encouraging and reinforce our belief that the Moderna COVID-19 Vaccine should remain protective against newly detected variants,” Stéphane Bancel, chief executive officer of Moderna, said in a statement. Currently, little data has been released showing just how effective the Johnson & Johnson is at protecting against the Delta variant, though it is believed that the single-shot vaccine does offer protection against the variant. Dr. Scott Gottlieb, former Food and Drug Administration commissioner, reportedly said the Johnson & Johnson vaccine appears to be about 60% effective against the Delta variant. Still, medical experts say any of the three vaccines currently being used in the U.S. continue to show good results as far as protection. ”This will protect them against getting very sick and being hospitalized and even dying from the Delta variant,” Dr. Katherine Gergen-Barnett of Boston Medical Center recently told NBC10 Boston. Will a booster shot be needed? So far, there has been no recommendation from the Centers for Disease Control and Prevention surrounding booster shots with the Delta variant. Still, health experts have repeatedly cautioned that COVID-19 booster shots could be needed for fully vaccinated people, particularly as new variants spread. White House chief advisor Dr. Anthony Fauci said during an interview with MSNBC's Medhi Hasan in April that people may need to get booster shots in a year. Pfizer CEO Albert Bourla also previously said people will "likely" need a third dose within 12 months of getting fully vaccinated. So far, studies suggest that the vaccines currently in use can recognize the emerging variants — but they may not provide quite as much protection against the new strains. Boosters and new versions of vaccines that target the variants are already being explored. Pfizer-BioNTech was previously testing a third booster shot of its vaccine on fully vaccinated people. "The flexibility of our proprietary mRNA vaccine platform allows us to technically develop booster vaccines within weeks, if needed," Sahin said in a release in February. Moderna was also testing a potential third dose of its current vaccine, and a possible booster shot specifically targeting the South Africa variant. Citing early data, the company recently said the booster vaccine generated a promising immune response against the B.1.351 and P.1 variants first identified in South Africa and Brazil, respectively. Meanwhile, Johnson & Johnson CEO Alex Gorsky said during an interview with CNBC's "Squawk Box" in March that the company is well-positioned to adapt its vaccine for variants, and is working on developing software that will "help address some of these new and emerging variants."

|
Scooped by
Juan Lama
|
Data have demonstrated vaccine protects against COVID-19 related hospitalization in broad geographic regions, including those with emerging variants. Decision follows the European Medicines Agency recommendation of the J&J COVID-19 vaccine. The Company aims to begin delivery of its vaccine to the EU in the second half of April and is committed to supply 200 million doses in 2021. NEW BRUNSWICK, N.J., March 11, 2021 – Johnson & Johnson (NYSE: JNJ) (the Company) today announced that the European Commission (EC) has granted a Conditional Marketing Authorization (CMA) for its single-dose COVID-19 vaccine, developed by the Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen), to prevent COVID-19 in individuals 18 years of age and older. The CMA follows a Positive Opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP). The CMA is valid in all 27 member states of the European Union (EU), plus Norway, Iceland and Liechtenstein. Data from the Phase 3 ENSEMBLE study showed that the Johnson & Johnson COVID-19 vaccine was well tolerated and demonstrated a 67 percent reduction in symptomatic COVID-19 disease in participants who received the vaccine in comparison to participants given the placebo. The onset of protection was observed from day 14 and was maintained 28 days post-vaccination.1 The data also demonstrated the vaccine was 85 percent effective in preventing severe disease across all regions studied, and showed protection against COVID-19 related hospitalisation and death, beginning 28 days after vaccination. “For more than a year, we have been working around the clock – leveraging the scientific minds, scale and resources of our global organisation to bring forward a COVID-19 vaccine,” said Alex Gorsky, Chairman and Chief Executive Officer at Johnson & Johnson. “We are thrilled with today’s Conditional Marketing Authorization by the European Commission, which enables our single-dose vaccine to reach many more communities in need, as we continue to do everything we can to help bring an end to this pandemic.” Johnson & Johnson is committed to making its COVID-19 vaccine available on a not-for-profit basis for emergency pandemic use. The Company aims to begin delivery of its single dose COVID-19 vaccine to the EU in the second half of April and to supply 200 million doses to the EU, plus Norway and Iceland in 2021. “This vaccine is the result of more than a decade of investment in research and development and deep commitment by our scientists. We appreciate the collaboration and the support of the European Commission in this monumental effort,” said Paul Stoffels, M.D., Vice Chairman of the Executive Committee and Chief Scientific Officer at Johnson & Johnson. “With this Conditional Marketing Authorization, we are proud to bring our single-shot vaccine to help protect millions of people across EU member states.” In December 2020, the Company announced that Janssen initiated a rolling submission with the EMA for its single-dose COVID-19 vaccine candidate, enabling an expedited CHMP review process. The COVID-19 vaccine candidate has also been filed for an Emergency Use Listing (EUL) with the World Health Organization. Rolling submissions for our vaccine candidate have also been initiated in several countries worldwide. “This latest major regulatory milestone would not have been possible without the hard work and dedication of everyone involved in our COVID-19 vaccine clinical trial programme, including our J&J team, our partners and study participants,” said Mathai Mammen, M.D., Ph.D., Global Head, Janssen Research & Development, Johnson & Johnson. “We are delighted by today’s announcement and remain fully committed to continuing our COVID-19 vaccine clinical programme as we strive to provide our single-dose COVID-19 vaccine to people all over the world.” The Company received Emergency Use Authorization (EUA) in the United States on February 27, following a unanimous vote by the U.S. Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee on February 26, 2021. The Johnson & Johnson single-dose COVID-19 vaccine has also been granted Interim Order authorisation in Canada. EMA Press Release (March 11, 2021): https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-janssen-authorisation-eu
|

|
Scooped by
Juan Lama
|
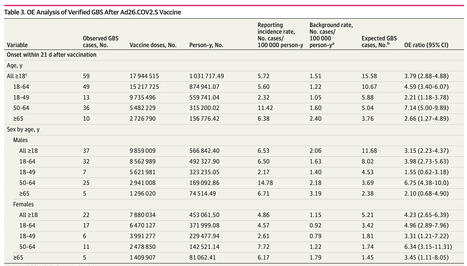
Question Are Ad26.COV2.S (Janssen), BNT162b2 (Pfizer-BioNTech), or mRNA-1273 (Moderna) COVID-19 vaccines associated with Guillain-Barré syndrome (GBS) within 21 or 42 days after vaccination? Findings This cohort study of 487 651 785 COVID-19 vaccine doses found that in observed-to-expected analyses, the observed number of GBS reports was higher than expected based on background rates within 21 and 42 days after vaccination for Ad26.COV2.S but not BNT162b2 or mRNA-1273. GBS reporting rates within 21 and 42 days of Ad26.COV2.S vaccination were 9 to 12 times higher than after BNT162b2 or mRNA-1273 vaccination. Meaning These findings suggest that Ad26.COV2.S vaccination was associated with GBS and that GBS after BNT162b2 and mRNA-1273 may represent background incidence. Importance Because of historical associations between vaccines and Guillain-Barré syndrome (GBS), the condition was a prespecified adverse event of special interest for COVID-19 vaccine monitoring. Objective To evaluate GBS reports to the Vaccine Adverse Event Reporting System (VAERS) and compare reporting patterns within 21 and 42 days after vaccination with Ad26.COV2.S (Janssen), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) COVID-19 vaccines. Design, Setting, and Participants This retrospective cohort study was conducted using US VAERS reports submitted during December 2020 to January 2022. GBS case reports verified as meeting the Brighton Collaboration case definition for GBS in US adults after COVID-19 vaccination were included. Exposures Receipt of the Ad26.COV2.S, BNT162b2, or mRNA-1273 COVID-19 vaccine. Main Outcomes and Measures Descriptive analyses of GBS case were conducted. GBS reporting rates within 21 and 42 days after Ad26.COV2.S, BNT162b2, or mRNA-1273 vaccination based on doses administered were calculated. Reporting rate ratios (RRRs) after receipt of Ad26.COV2.S vs BNT162b2 or mRNA-1273 within 21- and 42-day postvaccination intervals were calculated. Observed-to-expected (OE) ratios were estimated using published GBS background rates. Results Among 487 651 785 COVID-19 vaccine doses, 17 944 515 doses (3.7%) were Ad26.COV2.S, 266 859 784 doses (54.7%) were BNT162b2, and 202 847 486 doses (41.6%) were mRNA-1273. Of 295 verified reports of individuals with GBS identified after COVID-19 vaccination (12 Asian [4.1%], 18 Black [6.1%], and 193 White [65.4%]; 17 Hispanic [5.8%]; 169 males [57.3%]; median [IQR] age, 59.0 [46.0-68.0] years), 275 reports (93.2%) documented hospitalization. There were 209 and 253 reports of GBS that occurred within 21 days and 42 days of vaccination, respectively. Within 21 days of vaccination, GBS reporting rates per 1 000 000 doses were 3.29 for Ad26.COV.2, 0.29 for BNT162b2, and 0.35 for mRNA-1273 administered; within 42 days of vaccination, they were 4.07 for Ad26.COV.2, 0.34 for BNT162b2, and 0.44 for mRNA-1273. GBS was more frequently reported within 21 days after Ad26.COV2.S than after BNT162b2 (RRR = 11.40; 95% CI, 8.11-15.99) or mRNA-1273 (RRR = 9.26; 95% CI, 6.57-13.07) vaccination; similar findings were observed within 42 days after vaccination (BNT162b2: RRR = 12.06; 95% CI, 8.86-16.43; mRNA-1273: RRR = 9.27; 95% CI, 6.80-12.63). OE ratios were 3.79 (95% CI, 2.88-4.88) for 21-day and 2.34 (95% CI, 1.83-2.94) for 42-day intervals after Ad26.COV2.S vaccination and less than 1 (not significant) after BNT162b2 and mRNA-1273 vaccination within both postvaccination periods. Conclusions and Relevance This study found disproportionate reporting and imbalances after Ad26.COV2.S vaccination, suggesting that Ad26.COV2.S vaccination was associated with increased risk for GBS. No associations between mRNA COVID-19 vaccines and risk of GBS were observed.

|
Scooped by
Juan Lama
|
Vaccine advisers to the US Food and Drug Administration voted unanimously Friday to recommend a booster dose of Johnson & Johnson's vaccine at least two months after people get the first dose. The FDA's Vaccines and Related Biological Products Advisory Committee voted 19-0 to recommend the extra dose for all recipients of the J&J Janssen vaccine, 18 and older. They asked to simplify the original question being posed by the FDA, which had asked the committee to say whether the data showed that waiting six months or longer after getting the first shot would provide an even stronger immune response. The FDA will now consider the committee's advice. Then the US Centers for Disease Control and Prevention's vaccine advisers will be asked to consider it. Johnson & Johnson says studies have shown boosting at two or six months can bring that effectiveness up to 94% and it says its effectiveness does not wane over time in the same way that effectiveness from Pfizer's vaccine does. But the Johnson & Johnson vaccine has not been shown to be as protective as either the Pfizer or Moderna vaccines, noted VRBPAC chair Dr. Arnold Monto, a professor of public health and epidemiology at the University of Michigan. "So there is some urgency here to do something," he told the meeting. And the CDC's Dr. Amanda Cohn told the meeting that various studies suggested real-world efficacy of J&J's vaccine was anywhere between 50% and 68%. "Regardless of whether or not there been waning or this is the true effectiveness after a single dose, the effectiveness or protection of a single dose of the J&J vaccine is not equivalent to protection at this time with either two doses of an mRNA vaccine and certainly not in those groups who have now been authorized to receive a booster dose of an mRNA vaccine," Cohn said. Members of the committee said while there was not much data to show whether the efficacy of the Janssen vaccine was waning -- or was strong to begin with -- they agreed people should be given the opportunity for a booster. "I would say I agree a second dose booster is needed to boost immunity back to the 90-plus range," Dr. Archana Chatterjee, a pediatric infectious diseases expert at Rosalind Franklin University in Chicago, said before the vote. The FDA has already given EUA to a booster for Pfizer's vaccine for people who are six months out from their first two shots who are also either 65 or older or who are at least 18 and have a higher risk of severe disease because of pre-existing conditions or because of work or living conditions. And Americans are already flocking to get those boosters. Data from the US Centers for Disease Control and Prevention show close to 5% of fully vaccinated people -- about 9 million people -- have received booster shots. On Thursday, VRBPAC members voted unanimously to recommend booster doses of Moderna's vaccine to the same groups. If the FDA gives emergency use authorization to Moderna or Johnson & Johnson boosters, CDC vaccine advisers will meet to discuss which groups to recommend them to. Typically, shots can be administered once the CDC director signs off on the recommendation. CDC's Advisory Committee on Immunization Practices is scheduled to discuss boosters on October 21.

|
Scooped by
Juan Lama
|
Johnson & Johnson said its Covid vaccine was effective against the highly contagious Delta variant, adding to the growing body of evidence that the most widely available Covid shots offer protection against its most dangerous variants. Even eight months after inoculation, the single-shot J.&J. vaccine is proving to be highly effective against Delta, the company reported on Thursday, a reassuring finding for the 11 million Americans who have gotten the shot and for countries around the world betting on receiving the vaccine. In the United States, the variant, first identified in India, now accounts for an estimated one in four new cases, and the C.D.C. has listed it in 23 states. Johnson & Johnson said its vaccine showed a small drop in potency against the Delta variant, compared with its effectiveness against the original virus, and a larger drop against the Beta variant first identified in South Africa. That is the same pattern seen with the mRNA vaccines made by Pfizer-BioNTech and Moderna. The intense discourse about Delta’s threat has left some people who are vaccinated feeling anxious about whether they are protected. The variant’s global spread has prompted new restrictions from Ireland to Malaysia. Frustration had been building about the lack of clarity around the Johnson & Johnson vaccine’s efficacy against Delta. And reports of a cluster of cases among players on the Yankees baseball team who had received the Johnson & Johnson shot, though all asymptomatic or mild, did nothing to assuage fears. Studies have shown that the Delta and Beta variants slightly lower the efficacy of the Pfizer and Moderna vaccines. For Pfizer, studies show that two doses offer 88 percent protection against the Delta variant, just below the 93 percent protection against Alpha. The Moderna vaccine has performed similarly to Pfizer’s in earlier studies. Johnson & Johnson has collected less data than its peers on the vaccines, and the study released on Thursday was small and has not yet been published in a scientific journal. Updates on the efficacy of the Johnson & Johnson vaccine have been slow because it was rolled out later than the Pfizer and Moderna vaccines in the United States. The vaccine offered about 72 percent protection against early versions of the virus. Research cited available at bioRxiv (July 1, 2021): https://www.biorxiv.org/content/10.1101/2021.07.01.450707v1

|
Scooped by
Juan Lama
|
The doses, produced at a Baltimore factory under federal review, could have been contaminated. Federal regulators have told Johnson & Johnson that about 60 million doses of its coronavirus vaccine produced at a troubled Baltimore factory cannot be used because of possible contamination, according to people familiar with the situation. The Food and Drug Administration plans to allow about 10 million doses to be distributed in the United States or sent to other countries, but with a warning that regulators cannot guarantee that Emergent BioSolutions, the company that operates the plant, followed good manufacturing practices. The agency has not yet decided whether Emergent can reopen the factory, which has been closed for two months because of regulatory concerns, the people said. The Johnson & Johnson doses administered in the United States so far were manufactured at the firm’s plant in the Netherlands, not by Emergent. For weeks the F.D.A. has been trying to figure out what to do about at least 170 million doses of vaccine that were left in limbo after the discovery of a major production mishap involving two vaccines manufactured at the Baltimore factory. More than 100 million doses of Johnson & Johnson and at least 70 million doses of AstraZeneca were put on hold after Emergent discovered in March that its workers had contaminated a batch of Johnson & Johnson’s vaccine with a key ingredient used to produce AstraZeneca’s. Federal officials then ordered the plant to pause production, stripped Emergent of its responsibility to produce AstraZeneca’s vaccine and instructed Johnson & Johnson to assert direct control over the manufacturing of its vaccine there. Johnson & Johnson’s vaccine was once considered a potential game-changer in the nation’s vaccine stock because it required only one shot and was particularly useful in vulnerable communities. But the federal government now has an ample supply of the vaccines from Pfizer-BioNTech and Moderna, the two other federally authorized vaccine developers, and no longer needs Johnson & Johnson’s supply. Still, the loss of 60 million Johnson & Johnson doses puts a dent in the Biden administration’s plan to distribute vaccines to other countries that are still in the grip of the pandemic. The administration had been counting on sharing doses of both Johnson & Johnson and AstraZeneca but had to delay its plan while the F.D.A. completed a review of the facility. After he arrived in Britain for the Group of 7 summit this week, President Biden announced he had found another source for donations. Pfizer-BioNTech has now agreed to sell his administration 500 million doses at cost for donation to low and lower-middle income countries over the next year. The World Health Organization estimates that 11 billion doses are needed globally to stamp out the epidemic.

|
Scooped by
Juan Lama
|
(Reuters) - GlaxoSmithKline Plc said an injection of its cabotegravir drug given every two months, in combination with Johnson & Johnson’s rilpivirine, was recommended for approval to treat HIV infections by a panel of the European health regulator. The treatment is a long-acting regimen, which can reduce the number of doses required to 12 or six per year instead of a daily intake of pills. If approved, it would be the first complete long-acting HIV regimen requiring dosing just once in two months (in the EU region), GSK said. The combination as an injection is already approved in Canada and is currently under review in the United States. Shares of GSK were trading up 2.5% at 1410.4p, while J&J shares were marginally up during premarket trading. Gilead’s Truvada daily oral pill is currently the standard of care for preventing HIV, but GSK hopes to challenge its dominance by making shorter, long-lasting regimens and less toxic alternatives through its ViiV Healthcare unit. The positive recommendation from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency comes months after GSK said cabotegravir was found to be 66% more effective in preventing HIV infections than Truvada. Some 75 million people worldwide have been infected with HIV and about 32 million people have died since it began in the 1980s. While final approvals are up to the European Commission, it generally follows the CHMP’s recommendation and endorses them within a couple of months. GSK public announcement (Oct. 16, 2020): https://www.gsk.com/en-gb/media/press-releases/viiv-healthcare-receives-positive-chmp-opinion-for-long-acting-regimen-for-the-treatment-of-hiv/
|



 Your new post is loading...
Your new post is loading...