Gene therapy that alters hemoglobin genes may be an answer to curing sickle cell disease (SCD) and beta thalassemia. These two common life-threatening anemias afflict millions of individuals across the globe. Scientists at St. Jude Children's Research Hospital and the Broad Institute of MIT and Harvard used a next-generation genome editing technology, adenosine base editing, to restart fetal hemoglobin expression in SCD patient cells. The approach raised the expression of fetal hemoglobin to higher, more stable, and more uniform levels than other genome editing technologies that use CRISPR/Cas9 nuclease in human hematopoietic stem cells. The findings were published in Nature Genetics.
Research and publish the best content.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
Already have an account: Login
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
|
|




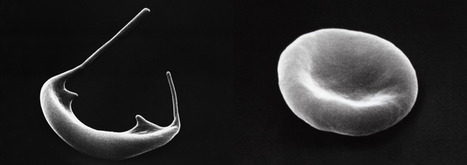







Gene therapy that alters hemoglobin genes may be an answer to curing sickle cell disease (SCD) and beta thalassemia. These two common life-threatening anemias afflict millions of individuals across the globe. Scientists at St. Jude Children's Research Hospital and the Broad Institute of MIT and Harvard used a next-generation genome editing technology, adenosine base editing, to restart fetal hemoglobin expression in SCD patient cells. The approach raised the expression of fetal hemoglobin to higher, more stable, and more uniform levels than other genome editing technologies that use CRISPR/Cas9 nuclease in human hematopoietic stem cells. The findings were published in Nature Genetics.