Researchers from Utah State University and Germany’s Helmholtz Institute for RNA-based Infection Research demonstrate that upon recognition of an RNA target, Cas12a2 cleaves all the other nucleic acids present, destroying the bacterial cell and preventing bacteriophage from replicating.
Research and publish the best content.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
Already have an account: Login
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
|
|




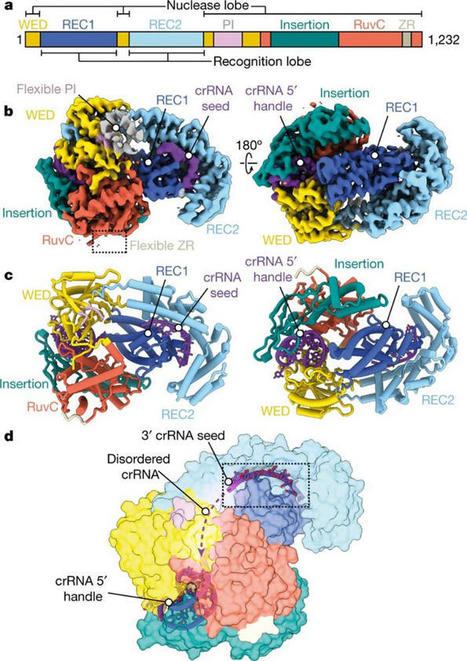







Despite its phylogenetic placement with DNA-targeting nucleases, Cas12a2 targets and cleaves RNA. When researchers put Cas12a2 in test tubes with pure DNA and a guide, nothing happened, because its target was not present and it remained inactive. However, sometimes a small amount of activating RNA was present, contaminating the sample. When the RNA was present and Cas12a2 recognized its target, it destroyed all nucleic acids in the tube. Unlike the CRISPR-Cas9 system, when Cas12a2 finds its target, the infected cell(s) die. This mechanism is known as abortive infection. One of the obvious applications of Cas12a2 is in CRISPR diagnostics. It can easily be reprogrammed to detect certain targets, such as RNA viruses, with high specificity. The researchers have already demonstrated the feasibility of this approach in their recent work. Cas12a2 could also be programmed to kill specific cell types, such as tumor cells, for therapeutic purposes.