Your new post is loading...

|
Scooped by
Juan Lama
|
RetroVirox offers a menu of cell-based antiviral services to evaluate experimental therapies and vaccines against coronaviruses, including SARS-CoV-2. The company offers in vitro testing with SARS-CoV-2 pseudovirions and with live SARS-CoV-2 viruses to evaluate entry inhibitors, neutralizing antibodies, and antivirals against the novel coronavirus causative agent of COVID-19. Multiple viral strains are available for testing, including the most recent Omicron variants of concern (XBB.1.5, XBB.1.16, XBB.2.3.2, EG.5.1 JN.1, KP.2, and KP.3). Pseudoviruses coated with the viral spike (S) protein of SARS-CoV-2 are also used to recapitulate the mode of entry of the novel coronavirus. Over 50 spike mutant and variants are available as pseudoviruses. These assays can be used for the following purposes: - To determine the neutralizing activity of therapeutic antibodies and antisera
- To test experimental COVID-19 vaccines using antisera from inoculated animals or humans
- To evaluate small-molecule and other entry inhibitors targeting the S viral protein, the ACE-2 viral receptor, or host proteases and other targets involved in SARS-CoV-2 viral entry
Assays with live replicating SARS-CoV-2, and milder forms of seasonal human coronaviruses (hCoV-OC43 and 229E) allow for the evaluation of inhibitors at all stages of the coronavirus life cycle. Additional Information about the coronavirus assays and many other cell-based antiviral assays offered at RetroVirox is available here. Request additional information by email at info@retrovirox.com

|
Scooped by
Juan Lama
|
A new vaccine for dengue received prequalification from the World Health Organization (WHO) on 10 May 2024. TAK-003 is the second dengue vaccine to be prequalified by WHO. Developed by Takeda, it is a live-attenuated vaccine containing weakened versions of the four serotypes of the virus that cause dengue. WHO recommends the use of TAK-003 in children aged 6–16 years in settings with high dengue burden and transmission intensity. The vaccine should be administered in a 2-dose schedule with a 3-month interval between doses. “The prequalification of TAK-003 is an important step in the expansion of global access to dengue vaccines, as it is now eligible for procurement by UN agencies including UNICEF and PAHO,” said Dr Rogerio Gaspar, WHO Director for Regulation and Prequalification. “With only two dengue vaccines to date prequalified, we look forward to more vaccine developers coming forward for assessment, so that we can ensure vaccines reach all communities who need it.” The WHO prequalification list also includes CYD-TDV vaccine against dengue developed by Sanofi Pasteur. Dengue is a vector-borne disease transmitted by the bite of an infected mosquito. Severe dengue is a potentially lethal complication which can develop from dengue infections. It is estimated that there are over 100-400 million cases of dengue worldwide each year and 3.8 billion people living in dengue endemic countries, most of which are in Asia, Africa, and the Americas. The largest number of dengue cases reported was in 2023 with the WHO Region of the Americas reporting 4.5 million cases and 2300 deaths. Dengue cases are likely to increase and expand geographically due to climate change and urbanization.

|
Scooped by
Juan Lama
|
CHICAGO, May 14 (Reuters) - U.S. government officials have temporarily relaxed strict guidelines on how public health laboratories and healthcare facilities handle, store and transport H5N1 bird flu samples, which are considered high-risk pathogens, in response to the recent spread of the virus to dairy cattle. The revised guidance, which has not been previously reported, came at the request of the Association of Public Health Laboratories (APHL), which represents state and local labs that monitor and detect public health threats, according to interviews and correspondence seen by Reuters. The H5N1 virus has been detected among dairy cattle in nine U.S. states since late March. The threat to the general public is still considered low, although dairy workers have been instructed to take extra safety precautions. APHL Executive Director Scott Becker said his group made the request to prepare for the possibility that H5N1 avian influenza, or bird flu, acquires the ability to become easily transmitted among people. The U.S. government strictly regulates the handling of so-called select agents, which include H5N1, Ebola, ricin and anthrax. All select agent material typically must be destroyed, decontaminated or transferred to a registered select agent facility within seven days of notification. Under the exemption, opens new tab ordered by the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service, labs handling samples identified as highly pathogenic avian influenza have a month to perform many of those tasks, reducing the bureaucratic burden and allowing lab staff to focus on testing, Becker said. The guidance changes, which took effect on May 3, modify requirements for handling H5N1 under federal select agent and toxin regulations for a period of 180 days. They apply to state and local government-run public health labs as well as labs that handle wastewater specimens, which are being used to help track the virus. "This is one of those lessons learned from COVID," Becker said, referring to pandemic preparedness plans set in place after the COVID-19 pandemic, during which labs were scrambling to respond after a disastrously slow start to testing. Exemption (May 3, 2024):

|
Scooped by
Juan Lama
|
Genetic analysis of 50,000-year-old Neanderthal skeletons has uncovered the remnants of three viruses related to modern human pathogens, and the researchers think they could be recreated. Genetic sequences from three common viruses that plague humanity today have been isolated from the remains of Neanderthals who lived more than 50,000 years ago. Marcelo Briones at the Federal University of São Paulo, Brazil, says it may be possible to synthesise these viruses and infect modern human cells with them in the lab... Preprint of the study in bioRxiv: https://doi.org/10.1101/2023.03.16.532919

|
Scooped by
Juan Lama
|
Federal public health officials are turning to wastewater surveillance to help fill in the gaps in efforts to track H5N1 bird flu outbreaks in dairy cows. R eluctance among dairy farmers to report H5N1 bird flu outbreaks within their herds or allow testing of their workers has made it difficult to keep up with the virus’s rapid spread, prompting federal public health officials to look to wastewater to help fill in the gaps. On Tuesday, the Centers for Disease Control and Prevention is expected to unveil a public dashboard tracking influenza A viruses in sewage that the agency has been collecting from 600 wastewater treatment sites around the country since last fall. The testing is not H5N1-specific; H5N1 belongs to the large influenza A family of viruses, as do two of the viruses that regularly sicken people during flu season. But flu viruses that cause human disease circulate at very low levels during the summer months. So the presence of high levels of influenza A in wastewater from now through the end of the summer could be a reliable indicator that something unusual is going on in a particular area. Wastewater monitoring, at least at this stage, cannot discern the sources — be they from dairy cattle, run-off from dairy processors, or human infections — of any viral genetic fragments found in sewage, although the agency is working on having more capability to do so in the future. CDC wastewater team lead Amy Kirby told STAT that starting around late March or early April, some wastewater collection sites started to notice unusual increases in influenza A virus in their samplings. Those readings stood out because by the last week of March, data the CDC tracks on the percentage of people seeking medical care for influenza-like illnesses suggested that the 2023-2024 flu season was effectively over. The increases were very site-specific, she said, and were not reflected in other areas. In fact, she called it “a very limited phenomenon. … The vast majority of our sites are not seeing this.” To date there has been little information in the public sphere about where infected cattle herds have been located. When the U.S. Department of Agriculture announces positive test results, it merely names the state in which the herd was located when the testing took place. Last week, the USDA reported that six additional herds — in Michigan, Idaho, and Colorado — had tested positive for H5N1, bringing the total to 42....

|
Scooped by
Juan Lama
|
The coronavirus disease 2019 (COVID-19) pandemic, along with the implementation of public health and social measures (PHSMs), have markedly reshaped infectious disease transmission dynamics. We analysed the impact of PHSMs on 24 notifiable infectious diseases (NIDs) in the Chinese mainland, using time series models to forecast transmission trends without PHSMs or pandemic. Our findings revealed distinct seasonal patterns in NID incidence, with respiratory diseases showing the greatest response to PHSMs, while bloodborne and sexually transmitted diseases responded more moderately. 8 NIDs were identified as susceptible to PHSMs, including hand, foot, and mouth disease, dengue fever, rubella, scarlet fever, pertussis, mumps, malaria, and Japanese encephalitis. The termination of PHSMs did not cause NIDs resurgence immediately, except for pertussis, which experienced its highest peak in December 2023 since January 2008. Our findings highlight the varied impact of PHSMs on different NIDs and the importance of sustainable, long-term strategies, like vaccine development. Public health and social measures for COVID-19 also impacted the incidence of other infectious diseases. In this study, the authors characterise the impacts of these measures on 24 notifiable infectious diseases in China until December 2023. Published in Nature Comm. (May 8, 2024): https://doi.org/10.1038/s41467-024-48201-8

|
Scooped by
Juan Lama
|
The alert comes after a man diagnosed with Crimean-Congo haemorrhagic fever (CCHF) was hospitalised, pushing the health officials to generate an alert. British tourists and expats in Spain have been issued an urgent warning about a deadly virus that kills 40 percent of the infected ones The alert comes after a man diagnosed with Crimean-Congo haemorrhagic fever (CCHF) was hospitalised, pushing the health officials to generate an alert. The Spanish Castile and León Ministry of Health confirmed that the patient is in a critical but stable condition. The disease, typically transmitted by ticks, is clinically associated with several symptoms, health bodies have revealed. Medical experts at Travel Health Pro told GB news: “The patient remains admitted, stable in serious conditions, at the Salamanca Hospital, where the protocolized epidemiological and care measures have been adopted.” The confirmed case is an elderly man, who has been admitted to hospital in Salamanca Hospital, in Northwestern Spain. “He has a tick bite and remains stable, although with the clinical severity that this pathology implies, with the isolation measures and protection of health professionals provided for these situations,” added Travel Health Pro. According to the WHO, the CCHF virus is transmitted to people either by tick bites or through contact with an infected animal's blood. The health body adds: “The majority of cases have occurred in people involved in the livestock industry, such as agricultural workers, slaughterhouse workers and veterinarians.” Anyone who has experienced symptoms or believes they may have been exposed to CCHF has been told to seek advice from a GP or NHS 111. “Remember - tell your healthcare providers that you travelled abroad,” said Travel Health Pro. In patients who recover from the disease, improvements tend to start around the ninth day or tenth day after the onset of illness. The WHO said: “It is difficult to prevent or control CCHF infection in animals and ticks as the tick-animal-tick cycle usually goes unnoticed and the infection in domestic animals is usually not apparent. “Furthermore, the tick vectors are numerous and widespread, so tick control with acaricides (chemicals intended to kill ticks) is only a realistic option for well-managed livestock production facilities. “There are no vaccines widely available for human or animal use. In the absence of a vaccine, the only way to reduce infection in people is by raising awareness of the risk factors and educating people about the measures they can take to reduce exposure to the virus.”

|
Scooped by
Juan Lama
|
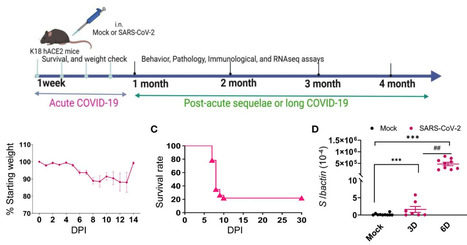
Viral variant is one known risk factor associated with post-acute sequelae of COVID-19 (PASC), yet the pathogenesis is largely unknown. Here, we studied SARS-CoV-2 Delta variant-induced PASC in K18-hACE2 mice. The virus replicated productively, induced robust inflammatory responses in lung and brain tissues, and caused weight loss and mortality during the acute infection. Longitudinal behavior studies in surviving mice up to 4 months post-acute infection revealed persistent abnormalities in neuropsychiatric state and motor behaviors, while reflex and sensory functions recovered over time. In the brain, no detectable viral RNA and minimal residential immune cell activation was observed in the surviving mice post-acute infection. Transcriptome analysis revealed persistent activation of immune pathways, including humoral responses, complement, and phagocytosis, and gene expression levels associated with ataxia telangiectasia, impaired cognitive function and memory recall, and neuronal dysfunction and degeneration. Furthermore, surviving mice maintained potent systemic T helper 1 prone cellular immune responses and strong sera neutralizing antibodies against Delta and Omicron variants months post-acute infection. Overall, our findings suggest that infection in K18-hACE2 mice recapitulates the persistent clinical symptoms reported in long-COVID patients and provides new insights into the role of systemic and brain residential immune factors in PASC pathogenesis. Published in Frontiers in Immunology (May 2, 2024): https://doi.org/10.3389/fimmu.2024.1384516

|
Scooped by
Juan Lama
|
An outbreak of H5N1 highly pathogenic influenza A virus (HPIAV) has been detected in dairy cows in the United States. Influenza A virus (IAV) is a negative-sense, single-stranded, RNA virus that has not previously been associated with widespread infection in cattle. As such, cattle are an extremely under-studied domestic IAV host species. IAV receptors on host cells are sialic acids (SAs) that are bound to galactose in either an α2,3 or α2,6 linkage. Human IAVs preferentially bind SA-α2,6 (human receptor), whereas avian IAVs have a preference for α2,3 (avian receptor). The avian receptor can further be divided into two receptors: IAVs isolated from chickens generally bind more tightly to SA-α2,3-Gal-β1,4 (chicken receptor), whereas IAVs isolated from duck to SA-α2,3-Gal-β1,3 (duck receptor). We found all receptors were expressed, to a different degree, in the mammary gland, respiratory tract, and cerebrum of beef and/or dairy cattle. The duck and human IAV receptors were widely expressed in the bovine mammary gland, whereas the chicken receptor dominated the respiratory tract. In general, only a low expression of IAV receptors was observed in the neurons of the cerebrum. These results provide a mechanistic rationale for the high levels of H5N1 virus reported in infected bovine milk and show cattle have the potential to act as a mixing vessel for novel IAV generation. Preprint in bioRxiv (May 3, 2024): https://doi.org/10.1101/2024.05.03.592326

|
Scooped by
Juan Lama
|
Highly pathogenic avian influenza (HPAI) viruses cross species barriers and have the potential to cause pandemics. In North America, HPAI A(H5N1) viruses related to the goose/Guangdong 2.3.4.4b hemagglutinin phylogenetic clade have infected wild birds, poultry, and mammals. Our genomic analysis and epidemiological investigation showed that a reassortment event in wild bird populations preceded a single wild bird-to-cattle transmission episode. The movement of asymptomatic cattle has likely played a role in the spread of HPAI within the United States dairy herd. Some molecular markers in virus populations were detected at low frequency that may lead to changes in transmission efficiency and phenotype after evolution in dairy cattle. Continued transmission of H5N1 HPAI within dairy cattle increases the risk for infection and subsequent spread of the virus to human populations. Preprint in bioRxiv (May 01, 2024): https://doi.org/10.1101/2024.05.01.591751

|
Scooped by
Juan Lama
|
We report highly pathogenic avian influenza A(H5N1) virus in dairy cattle and cats in Kansas and Texas, United States, which reflects the continued spread of clade 2.3.4.4b viruses that entered the country in late 2021. Infected cattle experienced nonspecific illness, reduced feed intake and rumination, and an abrupt drop in milk production, but fatal systemic influenza infection developed in domestic cats fed raw (unpasteurized) colostrum and milk from affected cows. Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus–infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission. Published in Emerging Infectious Diseases (April 20, 2024) https://doi.org/10.3201/eid3007.240508

|
Scooped by
Juan Lama
|
The BEQVEZ gene therapy inserts a working copy of the Factor IX (FIX) gene that codes for a high-activity FIX variant. Patients with moderate to severe hemophilia B can now take Pfizer’s BEQVEZ (fidanacogene elaparvovec-dzkt) with the approval of the FDA. Hemophilia B is an inherited bleeding disorder that affects more than 38,000 people globally and is caused by impaired blood clotting due to a factor IX (FIX) deficiency. The current gold standard for FIX replacement therapy prevention is infusions given once weekly or monthly. These infusions supplement or temporarily replace low blood clotting factor levels. Despite receiving prophylaxis and regular intravenous infusions, people with moderate to severe hemophilia B may still experience spontaneous bleeding episodes. Additionally, the current standard of care places a burden on healthcare systems’ budgets and resource allocation...

|
Scooped by
Juan Lama
|
By cutting trees in response to international demand for tobacco, farmers induced wildlife to start eating virus-laden bat guano. Zoonotic diseases, or illnesses transmitted from animals to humans, account for about three quarters of new infectious diseases around the world, including some that could lead to pandemics. The risk of a pathogen jumping from an animal to a human increases when people encroach on ecosystems and cause relationships to be disrupted between species—but how that risk actually becomes a reality can be unpredictable and difficult to untangle. A new paper published this week in Communications Biology shines rare light on one such case study: an example showing how international demand for tobacco led to habitat alterations in Uganda that seemingly drove chimpanzees and other species to begin consuming bat guano for mineral nutrients. In that process, the animals might have been exposed to more than two dozen viruses, including a novel cousin of the COVID-causing pathogen SARS-CoV-2...
|

|
Scooped by
Juan Lama
|
The U.S. Food and Drug Administration has approved a kit that will allow women to collect their own vaginal sample for HPV screening, a move that could increase early detection in those at risk for cervical cancer. Women will be able to swab themselves in privacy at a doctor's office, clinic or pharmacy, and the sample will then be sent off for analysis. More than half of U.S. women diagnosed with cervical cancer have never been screened or screened only infrequently for the virus, the kit's maker Roche said in a news release announcing the approval on May 15 2024. "With vaccinations, innovative diagnostic tools and screening programs, achieving the WHO's goal of eliminating cervical cancer by 2030 is within reach," Roche Diagnostics CEO Matt Sause said. "Our HPV self-collection solution helps support this goal by reducing barriers and providing access to HPV screening by allowing people to privately collect their own sample for HPV testing." Each year, about 11,500 U.S. women are diagnosed with cervical cancer and about 4,000 women die from it, according to the U.S. Centers for Disease Control and Prevention. HPV is the known cause of more than 95% of cervical cancers, Roche added. "Almost all cervical cancers are caused by persistent infection with certain types of HPV," Dr. Karen Knudsen, CEO of the American Cancer Society (ACS), said in a statement. "Self-collection can expand access to screening and reduces barriers, which will give more people the opportunity to detect, treat and ultimately survive cancer." Most primary care doctors don't test for HPV. Instead, women are most often screened by gynecologists during a pelvic exam, a procedure to which some don't have access and others find too intrusive and embarrassing. "Roche's self-collection solution can help reduce these barriers by offering an alternative to clinician collection procedures, while also providing accurate and reliable results," Roche said. The HPV test itself is already covered by private insurance, Medicare and Medicaid, Roche told the Washington Post. "This literally just opens up another option for a different demographic of people that might not feel comfortable, that might not have access [and] may not have time" to get tested otherwise, Irene Aninye, chief science officer for the Society for Women's Health Research, told the Post. The ACS recommends that cervical cancer screening begin at age 25, and that women ages 25 to 65 have an HPV test every five years. Studies done for the past two decades have found that self-collection for HPV testing is feasible and acceptable, and that women can collect samples as well as their physicians, the ACS said. "Self-collection was not FDA-approved at the time our current guideline was released, but we now expect a minor update to the guideline to note that primary HPV testing via clinician-collected sample or self-collection is acceptable," said ACS Chief Scientific Officer Dr. William Dahut. "We anticipate self-collection will play an increasingly prominent role in cervical cancer screening once regulatory and clinical prerequisites are in place and as supporting evidence continues to accumulate," Dahut added. The approval could also open the door for at-home collection of samples. Teal Health received FDA breakthrough designation last week for an at-home cervical cancer screening device called the Teal Wand. Women would collect their own sample at home, then send it to a lab to be tested for HPV. The designation grants Teal Health priority status from regulators when clinical trials wrap up and the data is submitted to the FDA. "No more stirrups, no more speculum," the Teal Health website promises. "The Teal Wand replaces the need for an in-office pap smear using stirrups, a speculum and a hard plastic brush or broom. With self-collect, you are in control." Roche's Press release (May 14, 2024): https://www.roche.com/media/releases/med-cor-2024-05-15

|
Scooped by
Juan Lama
|
Scientists have found several strains of highly pathogenic avian influenza in a small number of NYC’s wild birds. A recent study has found a very small number of wild urban birds that either live in or migrate through New York City are carrying an unexpected hitchhiker: the highly pathogenic avian influenza virus H5N1 (ref). This worrying discovery demonstrates that potentially serious human health risks are not limited to rural environments or to commercial factory farms as most people believe, but can also be found lurking in large cities. Thus, this study — the first of its kind — highlights the dangers of close contact between animals and humans because they may end up sharing infections — or even pandemics. “To my knowledge, this is the first large-scale U.S. study of avian influenza in an urban area, and the first with active community involvement,” the study’s senior author, microbiologist and geneticist Christine Marizzi, an Adjunct Assistant Professor at the Icahn School of Medicine at Mount Sinai, said in a statement. Dr Marizzi also is the principal investigator of the New York City Virus Hunters (NYCVH) Program, and the director of community science at BioBus, which is an initiative to bring cutting-edge laboratory science to communities of students that have been historically underrepresented in science with the help of a converted school bus. Many migratory bird species stop over in New York City during their long journeys, which creates the ideal opportunity for different virus strains to meet and mix. “Birds are key to finding out which influenza and other avian viruses are circulating in the New York City area, as well as important for understanding which ones can be dangerous to both other birds and humans,” Dr Marizzi explained. “And we need more eyes on the ground — that’s why community involvement is really critical.” The findings were the result of a citizen scientist program to monitor the health of urban wild birds. Samples were collected by high school students in New York City who participate in both research and communication efforts as paid interns under Dr Marizzi’s expert mentorship. As part of their work, they’re provided with all the necessary protective gear to go out into the field and collect bird fecal samples, which they then help screen for viruses. Additional samples are also donated from local animal welfare centers, particularly the Wild Bird Fund, which is a local wild bird rescue. (Disclosure: I’ve been a $upporter of the Wild Bird Fund for many years.). To do this study, the junior scientist interns, or “Virus Hunters”, collected more than 2500 swabs and fecal samples in total from the Wild Bird Fund and from NYC’s parks and other natural areas across the boroughs of Manhattan, Brooklyn and the Bronx. The samples were then screened in a lab at the Icahn School of Medicine at Mount Sinai for the presence of two avian viruses: avian influenza and avian paramyxovirus. The Virus Hunters identified eight birds that were positive for avian paramyxovirus using Polymerase Chain Reaction (PCR). Two type 1 avian paramyxoviruses (APMV1, also known as Newcastle’s Disease Virus), were isolated from feral rock pigeons, Columba livia. These two viral isolates were named, reported to the USDA, and the whole genome sequences were published at the online data repository GenBank. APMV1 is a viral pathogen that affects pigeons and is present in most countries. It is a serious disease that can spread rapidly and cause high rates of pigeon illness and death. Other paramyxovirus strains may affect other avian species, particularly poultry. Between January 2022 and November 2023, the Virus Hunters continued their work by collecting 1927 samples from raptors, poultry and waterfowl (Figure 1). They screened these samples specifically for H5N1, also known as bird flu. They detected the virus in six city birds representing four different species: Canada geese, Branta canadensis; red-tailed hawks, Buteo jamaicensis; peregrine falcons, Falco peregrinus; and domestic chickens, Gallus gallus domesticus. The positive samples came from the urban wildlife rehabilitation centers, highlighting the critical role that such centers can play in viral surveillance. After comparing the genomic sequences of the samples to each other and to other H5N1 viral genomes available in GenBank, the Virus Hunters found they were a mix of the Eurasian H5N1 2.3.4.4.b clade and local North American avian influenza virus genotypes. Is New York City sitting on a ticking H5N1 pandemic time bomb? “It is important to mention that, because we found H5N1 in city birds, this does not signal the start of a human influenza pandemic,” Dr Marizzi cautioned. “We know that H5N1 has been around in New York City for about 2 years and there have been no human cases reported.” Currently, only one person has so far been infected by the H5N1 virus in the United States — a dairy worker in Texas. And yet despite the rarity of human H5N1 infections, this virus worries scientists that as bird flu spreads worldwide, the risk of it “jumping” the species barrier into humans continues to grow. For this reason, it’s important that people know what they can do to protect themselves from an infection with H5N1. “It’s smart to stay alert and stay away from wildlife,” Dr Marizzi advised. “This also includes preventing your pets from getting in close contact with wildlife.” Domestic cats are particularly susceptible. For example, out of 24 cats known to have contracted H5N1 on a single Texas dairy farm last month, twelve of them have died so far. If one must handle sick or injured birds or other wildlife, it is important to always use safe practices. These practices include placing a towel, blanket, jumper or pillowcase gently over the stricken animal. This will help keep it calm and aid with safe handling. If dealing with a sick or wounded bird, it is best to gently wrap it in a towel or hold the wings close to its body and place it into a paper bag or box for transport to bird rescue center. Research Published in Journal of Virology (May 15, 2024):

|
Scooped by
Juan Lama
|
A massive upsurge in dengue cases marked by multiple outbreaks is occurring worldwide and raising new questions about who is at elevated risk of severe forms of the mosquito-transmitted disease. Incidence of the infection has increased by orders of magnitude throughout the so-called dengue belt, which encompasses Central and South America, Sub-Saharan Africa, Southeast Asia and swaths of the South Pacific, home to densely populated islands. Dengue, without question, is the most widespread and rapidly increasing vector-borne disease in the world, according to the World Health Organization. In the Americas alone, more than 5.2 million cases have been documented and more than 1,000 deaths were reported within the first three months of 2024, the Pan American Health Organization reported in April, noting a marked surge over the same period in 2023. The story is similar in other dengue-affected areas of the world where lapses in vector control have conspired with global climate change to create an explosion of bloodthirsty mosquitoes, swarms of them moving into regions once considered dengue-free. Only female mosquitoes feed on blood, they're in constant need of the nutrients in it to nurture their eggs. Now, more than two decades of dengue surveillance in Thailand is answering a slew of questions at a time when the world needs guidance most. Findings from the research have revealed how various subgroups—what virologists call subtypes—of the dengue virus influence future risk of severe infection. It has been known for years that those who become infected in subsequent outbreaks, after a usually mild bout with a first-time infection, are at significant risk of severe disease in later dengue exposures. New research finally has analyzed more than 15,000 cases to discern why that is so. Writing in Science Translational Medicine, a global team of scientists has explained how the four dengue viral subtypes—DENV-1, 2, 3, and 4—influence the risk of repeated severe infections. The findings provide a new framework for disease monitoring and lay the foundation for vaccination strategies as the new dengue immunizations emerge. The team also underscored how dengue, a pernicious tropical malady, can be understood within the context of other common viral diseases that circle the globe. "The ability of viruses, such as SARS-CoV- 2 and influenza, to continuously change their genetic structure in response to the selective pressure of population immunity complicates control efforts," said Dr. Lin Wang, lead author of the dengue study. "In the case of dengue virus, an arbovirus that infects more than 100 million people each year, the situation is even more complex," Wang continued. "Individuals with high dengue virus antibody titers are protected from infection and developing severe disease. "However, individuals with sub-neutralizing antibody titers have been shown to have the highest risk of severe disease, through multiple hypothesized mechanisms including antibody-dependent enhancement," emphasized Wang, a researcher in the genetics department at the University of Cambridge in England. A dengue infection can be tricky. Some patients who have weathered an infection but get infected in a subsequent outbreak can have more severe symptoms the second time around. Yet, most research on repeat dengue infections has regarded each of the serotypes as no different from the other, Wang and colleagues contend, noting that an assessment of each serotype's genetic differences was needed to provide a clearer picture of potential risks. To develop that clearer picture, researchers studied each serotype in more than 15,000 patients' infections as a way to peel away much of the mystery surrounding why first-time dengue illnesses are traditionally milder than subsequent ones. Working with Wang were collaborators from two centers in Bangkok, Thailand; multiple research institutes in the United States and one in France. To determine how each of the viral serotypes affects the risk of severe disease, Wang and colleagues analyzed viral genetic data. The team also studied cases of patients hospitalized for dengue to determine which viral subtype caused their infections. Researchers gathered data from 21 years of dengue surveillance, ranging from 1994 to 2014, in a children's hospital in Bangkok, encompassing 15,281 individual cases. This allowed them to find repeat cases and each viral subtype in all infections. Based on the pediatric patients' hospital records, researchers discovered a link between hospitalization and the order in which patients became infected with different dengue-virus serotypes. They were also able to determine which combinations of viral subtypes pointed to mild or severe forms of dengue. For instance, people who became infected with serotypes that were very similar, such as DENV-3 and DENV-4, or very different serotypes as in the case of DENV-1 and DENV-4, tended to have a lower risk of severe disease during the second infection. That said, patients who were infected with serotypes that were only moderately different had a higher risk of severe symptoms in subsequent infections. The highest risk group in this category involved patients who had an initial infection with DENV-2 followed by a subsequent infection triggered by DENV-1. The new research adds clarity to a disease risk that may seem paradoxical to the lay public. For example, most people infected with dengue virus for the first time develop extremely mild signs of the disease or none at all. But for those who do get sick, soaring fever, headache, body aches, nausea and rash are the primary symptoms, and they intensify in severe manifestations of the infection. For more than a century a severe bout with dengue has been known as breakbone fever because of the intensity of the pain and accompanying muscle spasms. The virus is carried in the tropics and subtropics by Aedes aegypti and Aedes albopictus mosquitoes, which are endemic in the dengue belt. But while the belt, which runs through latitudes 35-degrees North and 35-degrees South, has traditionally been home to dengue-carrying mosquitoes, the arthropods have been extending their range northward as global climate change intensifies, scientists say. Wang, meanwhile, reports that the collaborative research has set the stage to better understand immune system function in subsequent, severe dengue infections. "These findings suggest that immune imprinting helps determine dengue disease risk and provides a pathway to monitor the changing risk profile of populations and to quantifying risk profiles of candidate vaccines," Wang concluded. "This will become increasingly important as dengue vaccines begin to get used." Research published in Science Translational Medicine (April 24, 2024): https://doi.org/10.1126/scitranslmed.adk3259

|
Scooped by
Juan Lama
|
The patient is a soldier who had recently returned from abroad. People who may be at risk through contact with him are being traced. A case of Lassa fever - endemic to West Africa and spread by rats - has been reported in the Paris region. The patient, a soldier who had recently returned from abroad, is now in the Bégin military hospital in Saint-Mandé (Val-de-Marne). The Ministry of Health has said that an “in-depth epidemiological investigation is under way to determine the persons who may have been in contact with him”. It said his condition “does not give cause for concern”. What is Lassa fever? The fever takes its name from the town where it was first identified and isolated - Lassa in Nigeria, West Africa - and where it killed a nurse in 1969. It is now common in Nigeria, Guinea, Liberia, and Sierra Leone, and cases have also been reported in Cote d’Ivoire and Burkina Faso. There have been two epidemics in Benin in recent years; one in 2014 and another in 2016. The virus kills between 5,000 and 6,000 people per year in West Africa, and infects between 100,000 and 300,000 people. Its incubation period - from infection to symptoms - varies from two to 21 days. “The virus circulates almost constantly, especially in Nigeria, which is the worst-affected country,” said Sylvain Baize, head of the emerging viral infections unit at the Institut Pasteur, to BFMTV. “In all, it is estimated that 160 to 180 million people are potentially at risk.” How does Lassa fever spread? The virus is spread by rat faeces from the Natal rat (Mastomys natalensis), which is native to West Africa. The World Health Organization states: “The virus can also be transmitted from human to human by direct contact with the blood, urine, excrement or other bodily secretions of a contaminated person.” What are the symptoms of Lassa fever? In 80% of cases, it causes no symptoms. However, in the remaining 20%, symptoms come on gradually and become increasingly severe. They include fever, vomiting, nausea, stomach pain, and headaches. In 15% of cases, symptoms are worse, and include oedema (fluid build-up); pleurisy (inflammation of the lung lining); and oral, nasal or vaginal haemorrhage. The fever is fatal in around 1% of cases, as it goes on to cause organ failure. Of those who develop serious symptoms and do survive, some will have lasting heart problems, and 25% will become deaf. Only half of these will recover their hearing after one to three months. It is especially dangerous for pregnant women. What is the treatment for Lassa fever? So far, only one treatment has been identified. This is the antiviral agent ribavirin. This must be given very soon after infection to be effective. However, because the symptoms of the fever typically appear similar to other conditions (including malaria and dysentery), by the time the condition is confirmed, it is usually too late to give the drug. There is no vaccine yet available, although the Institut Pasteur released promising results to an international phase one trial in April. After-effects sometimes appear in those who survive this fever: 25% become deaf. Only half recover their hearing after one to three months. May 3, 2024

|
Scooped by
Juan Lama
|
Avian influenza (serotype H5N1) is a highly pathogenic virus that emerged in domestic waterfowl in 1996. Over the past decade, zoonotic transmission to mammals, including humans, has been reported. Although human to human transmission is rare, infection has been fatal in nearly half of patients who have contracted the virus in past outbreaks. The increasing presence of the virus in domesticated animals raises substantial concerns that viral adaptation to immunologically naive humans may result in the next flu pandemic. Wastewater-based epidemiology (WBE) to track viruses was historically used to track polio and has recently been implemented for SARS-CoV2 monitoring during the COVID-19 pandemic. Here, using an agnostic, hybrid-capture sequencing approach, we report the detection of H5N1 in wastewater in nine Texas cities, with a total catchment area population in the millions, over a two-month period from March 4th to April 25th, 2024. Sequencing reads uniquely aligning to H5N1 covered all eight genome segments, with best alignments to clade 2.3.4.4b. Notably, 19 of 23 monitored sites had at least one detection event, and the H5N1 serotype became dominant over seasonal influenza over time. A variant analysis suggests avian or bovine origin but other potential sources, especially humans, could not be excluded. We report the value of wastewater sequencing to track avian influenza. Preprint in medRxiv ( May 10, 2024): https://www.medrxiv.org/content/10.1101/2024.05.10.24307179v1.full.pdf

|
Scooped by
Juan Lama
|
The World Health Organization (WHO) was notified of three human cases, including one death, of Middle East respiratory syndrome coronavirus (MERS-CoV) between 10 and 17 April 2024, by the Ministry of Health of the Kingdom of Saudi Arabia (KSA). All three cases were males from Riyadh aged between 56 and 60 years with underlying health conditions and were not health care workers. The three cases are epidemiologically linked to exposures in a health-care facility in Riyadh, although investigations are ongoing to verify this and understand the route of transmission. Since the beginning of the year, a total of four cases and two deaths have been reported from the Kingdom of Saudi Arabia. The notification of these cases does not change WHO’s overall risk assessment, which remains moderate at both the global and regional levels....

|
Scooped by
Juan Lama
|
The avian influenza virus that has been infecting dairy cows and spreading alarm in the United States was expected to reach Germany this week. But that’s actually good news. A shipment of samples of the H5N1 virus from Cornell University virologist Diego Diel is destined for the Federal Research Institute for Animal Health in Riems, which has one of the rare high-security labs worldwide that are equipped to handle such dangerous pathogens in cattle and other large animals. There, veterinarian Martin Beer will use the samples to infect dairy cows, in search of a fuller picture of the threat the virus poses, to both cattle and people, than researchers have been able to glean from spotty data collected in the field. Six weeks into the outbreak that has spread to farms in nine U.S. states, the flow of data from those locations remains limited as public health officials sort out authorities and some farms resist oversight. “It’s incredibly difficult to get the right sample sets off the infected farms,” says Richard Webby, an avian influenza researcher at St. Jude Children’s Research Hospital. “It’s clearly a barrier to understanding what’s going on. … That’s why these experimental infections of cows are really going to be super informative.”

|
Scooped by
Juan Lama
|
Common diabetes drug lowers SARS-CoV-2 levels, clinical trial finds Stephanie Soucheray, MA COVID-19 Thinglass/iStock Share Today, researchers from the University of Minnesota published evidence that the common diabetes drug metformin decreases the amount of SARS-CoV-2 in the body and helps reduce the risk of rebound symptoms if given early in the course of non-severe illness. The study, published in Clinical Infectious Diseases, suggests metformin may also help prevent long COVID. The researchers tested metformin against a placebo in 999 adults infected with COVID-19. More than 50% of the study enrollees were vaccinated, and treatment took place when the Omicron variant was the most dominant strain in the United States. Study included those at standard-risk. Moreover, according to Carolyn Bramante, MD, principal investigator of the study and an assistant professor at the University of Minnesota, the study participants represented a standard- risk population, a group that currently lacks effective treatment options for the novel coronavirus. "This is not a high-risk population," Bramante told CIDRAP News. Instead, participants were 30 years or older, had a body mass index of 25 or higher (overweight), and did not require hospitalization for their COVID-19 infection. In several trials, Paxlovid has been shown to prevent deaths and hospitalization in high-risk, unvaccinated people, but standard-risk populations have not shown improvement in either time to resolution of symptoms or the incidence of hospitalization or death. Bramante said that these patient population demographics suggest metformin may be a clinical tool in outpatient medication that could be widely used. "The data support that someone would be justified if they prescribed it for outpatient treatment," she said. Four-fold reduction in viral load by day 10. Participants were given a 14-day course of metformin, and participants collected nasal swabs on days 1, 5, and 10. Bramante said early treatment was key: Participants were enrolled within 3 days of a positive test, and if symptomatic, reported having symptoms for 7 or fewer days. The mean SARS-CoV-2 viral load was reduced 3.6-fold with metformin relative to placebo by day 10, the authors found, and those who received metformin were less likely to have a detectable viral load than placebo at days 5 or 10 (odds ratio [OR], 0.72; 95% confidence interval [CI], 0.55 to 0.94). Metformin reduced the odds of hospitalization or death through 28 days by 58%; emergency department visits, hospitalizations, and death through 14 days by 42%; and long COVID through 10 months by 42%. Viral rebound, defined as a higher viral load at day 10 than day 5, was less frequent with metformin (3.28%) than placebo (5.95%; OR, 0.68; 95% CI, 0.36 to 1.29). While the mechanism of action is not known, Bramante said metformin likely lowers inflammation and inhibits translation of the virus. In a commentary on the study, the authors write, "This study makes a strong case for a potential effect of metformin on COVID-19 virologic decay and prompts reevaluation of existing data in support of its use. Research cited published (May 1, 2024) in Clin. Infect. Dis.: https://doi.org/10.1093/cid/ciae159

|
Scooped by
Juan Lama
|
Sporadic human infections with highly pathogenic avian influenza (HPAI) A(H5N1) virus, with a wide spectrum of clinical severity and a cumulative case fatality of more than 50%, have been reported in 23 countries over more than 20 years. HPAI A(H5N1) clade 2.3.4.4b viruses have spread widely among wild birds worldwide since 2020–2021, resulting in outbreaks in poultry and other animals. Recently, HPAI A(H5N1) clade 2.3.4.4b viruses were identified in dairy cows, and in unpasteurized milk samples, in multiple U.S. states. We report a case of HPAI A(H5N1) virus infection in a dairy farm worker in Texas. In late March 2024, an adult dairy farm worker had onset of redness and discomfort in the right eye. On presentation that day, subconjunctival hemorrhage and thin, serous drainage were noted in the right eye. Vital signs were unremarkable, with normal respiratory effort and an oxygen saturation of 97% while the patient was breathing ambient air. Auscultation revealed clear lungs. There was no history of fever or feverishness, respiratory symptoms, changes in vision, or other symptoms. The worker reported no contact with sick or dead wild birds, poultry, or other animals but reported direct and close exposure to dairy cows that appeared to be well and with sick cows that showed the same signs of illness as cows at other dairy farms in the same area of northern Texas with confirmed HPAI A(H5N1) virus infection (e.g., decreased milk production, reduced appetite, lethargy, fever, and dehydration). The worker reported wearing gloves when working with cows but did not use any respiratory or eye protection. Conjunctival and nasopharyngeal swab specimens were obtained from the right eye for influenza testing. The results of real-time reverse-transcription–polymerase-chain-reaction (RT-PCR) testing were presumptive for influenza A and A(H5) virus in both specimens. On the basis of a presumptive A(H5) result, home isolation was recommended, and oral oseltamivir (75 mg twice daily for 5 days) was provided for treatment of the worker and for postexposure prophylaxis for the worker’s household contacts (at the same dose). The next day, the worker reported no symptoms except discomfort in both eyes; reevaluation revealed subconjunctival hemorrhage in both eyes, with no visual impairment. Over the subsequent days, the worker reported resolution of conjunctivitis without respiratory symptoms, and household contacts remained well.... Published in NEJM (May 3, 2024):

|
Scooped by
Juan Lama
|
The USDA tested 30 samples from states with herds infected by H5N1. Tests of ground beef purchased at retail stores have been negative for bird flu so far, the U.S. Department of Agriculture announced Wednesday, after studying meat samples collected from states with herds infected by this year's unprecedented outbreak of the virus in cattle. The results "reaffirm that the meat supply is safe," the department said in a statement published late Wednesday after the testing was completed. Health authorities have cited the "rigorous meat inspection process" overseen by the department's Food Safety Inspection Service, or FSIS, when questioned about whether this year's outbreak in dairy cattle might also threaten meat eaters. "FSIS inspects each animal before slaughter, and all cattle carcasses must pass inspection after slaughter and be determined to be fit to enter the human food supply," the department said. The National Veterinary Services Laboratories tested 30 samples of ground beef in total, which were purchased at retail outlets in states with dairy cattle herds that had tested positive. To date, dairy cattle in at least nine states — Colorado, Idaho, Kansas, Michigan, New Mexico, North Carolina, Ohio, South Dakota and Texas — have tested positive for H5N1, which is often lethal to poultry and other animals, like cats, but has largely spared cattle aside from sometimes disrupting their production of milk for a few weeks. A USDA spokesperson said the ground beef they tested came from stores in only eight of those states. Colorado was only confirmed to have H5N1 in a dairy cow after USDA had collected the samples. The spokesperson did not comment on whether beef from stores in additional states would be sampled. More results from the department related to bird flu in beef are expected soon. Samples collected from the beef muscle of dairy cows condemned by inspectors at slaughter facilities are still being tested for the virus. The department is also testing how cooking beef patties to different temperatures will kill off the virus. "I want to emphasize, we are pretty sure that the meat supply is safe. We're doing this just to enhance our scientific knowledge, to make sure that we have additional data points to make that statement," Dr. Jose Emilio Esteban, USDA under secretary for food safety, told reporters Wednesday. The studies come after the USDA ramped up testing requirements on dairy cattle moving across state lines last month in response to the outbreak. Officials said that was in part because it had detected a mutated version of H5N1 in the lung tissue of an asymptomatic cow that had been sent to slaughter. While the cow was blocked from entering the food supply by FSIS, officials suggested the "isolated" incident raised questions about how the virus was spreading. Signs of bird flu have also made its way into the retail dairy supply, with as many as one in five samples of milk coming back positive in a nationwide Food and Drug Administration survey. The FDA has chalked those up to harmless fragments of the virus left over after pasteurization, pointing to experiments showing that there was no live infectious virus in the samples of products like milk and sour cream that had initially tested positive. But the discovery has worried health authorities and experts that cows could be flying under the radar without symptoms, given farms are supposed to be throwing away milk from sick cows. One herd that tested positive in North Carolina remains asymptomatic and is still actively producing milk, a spokesperson for the state's Department of Agriculture and Consumer Services told CBS News. It remains unclear how H5N1 has ended up in the milk supply. Don Prater, the FDA's top food safety official, said Wednesday that milk processors "can receive milk from hundreds of different farms, which may cross state lines," complicating efforts to trace back the virus. "This would take extensive testing to trace it that far," Bailee Woolstenhulme, a spokesperson for the Utah Department of Agriculture and Food, told CBS News. Woolstenhulme said health authorities are only able to easily trace back milk to so-called "bulk tanks" that bottlers get. "These bulk tanks include milk from multiple dairies, so we would have to test cows from all of the dairies whose milk was in the bulk tank," Woolstenhulme said.

|
Scooped by
Juan Lama
|
Virus has already killed other mammals including sea lions and seals, while also taking toll on farm animals. The first case of a walrus dying from bird flu has been detected on one of Norway’s Arctic islands, a researcher has said. The walrus was found last year on Hopen island in the Svalbard archipelago, Christian Lydersen, of the Norwegian Polar Institute, told AFP. Tests carried out by a German laboratory revealed the presence of bird flu, Lydersen said. The sample was too small to determine whether it was the H5N1 or the H5N8 strain. “It is the first time that bird flu has been recorded in a walrus,” Lydersen said. About six dead walrus were found last year in the Svalbard islands, about 1,000km (620 miles) from the north pole and halfway between mainland Norway and the north pole.

|
Scooped by
Juan Lama
|
Bird flu isn’t just lurking inside cows, new research shows. Florida scientists have reported the first known case of highly pathogenic H5N1 avian influenza in a common bottlenose dolphin (Tursiops truncatus). Though the case dates back to 2022, it’s the latest indication that these flu strains can potentially infect a wide variety of mammals. The report was published Friday in the Nature journal Communications Biology. According to the paper, the flu-infected dolphin was first identified on March 29, 2022. Researchers with the University of Florida’s Marine Animal Rescue Program were notified of a dolphin that appeared to be in clear distress around the waters of Horseshoe Beach in North Florida. By the time they arrived, however, the dolphin had already died. It was subsequently packed in ice and taken to the university for an autopsy the following day. The post-mortem examination found signs of poor health and inflammation in the dolphin’s brain and meninges (the membrane layers that protect the brain and spinal cord). The dolphin tested negative for other common infectious causes of brain inflammation, which prompted the researchers to expand their search. They knew that wild birds can develop neuroinflammation from highly pathogenic strains of avian influenza, that several bird die-offs tied to the flu had occurred in the area recently, and that recent outbreaks had occurred among other populations of marine mammals elsewhere, so they decided to screen for it. Testing then revealed the presence of H5N1 within the dolphin’s lungs and brain. There have been sightings of highly pathogenic avian influenza (HPAI) in other marine mammals lately, such as harbor seals and other species of dolphins. But this is the first reported case documented in a common bottlenose dolphin and the first report in any cetacean (dolphins and whales) within the waters of North America. Strains of bird flu are classified as highly pathogenic when they cause severe illness and deaths in wild birds, so it’s not necessarily a given that they will be as dangerous to other animals they infect. The current outbreaks of H5N1 bird flu in cows, for instance, have caused generally mild illness to date. But strains belonging to this particular lineage of H5N1 (2.3.4.4b) have been deadly to marine mammals. The strain in this case did not appear to develop known genetic changes that would make it easier to infect and transmit between mammals, the authors found. But flu viruses mutate very quickly, leaving open the possibility that some strains will adapt and pick up the right changes that can make them a much bigger threat to mammals both on land and in the sea. “Human health risk aside, the consequences of A(H5N1) viruses adapting for enhanced replication in and transmission between dolphins and other cetacea could be catastrophic for these populations,” the authors wrote. The researchers are continuing to investigate the case, hoping to pinpoint the origins of the dolphin’s infection and to better understand the potential for bird flu strains to successfully jump the species barrier to these marine mammals. Studiy cited published in Communications Biology (April 18, 2024): https://doi.org/10.1038/s42003-024-06173-x

|
Scooped by
Juan Lama
|
The ongoing evolution of SARS-CoV-2 to evade vaccines and therapeutics underlines the need for innovative therapies with high genetic barriers to resistance. Therefore, there is pronounced interest in identifying new pharmacological targets in the SARS-CoV-2 viral life cycle. The small molecule PAV-104, identified through a cell-free protein synthesis and assembly screen, was recently shown to target host protein assembly machinery in a manner specific to viral assembly. In this study, we investigate the capacity of PAV-104 to inhibit SARS-CoV-2 replication in human airway epithelial cells (AECs). We show that PAV-104 inhibits >99% of infection with diverse SARS-CoV-2 variants in immortalized AECs, and in primary human AECs cultured at the air-liquid interface (ALI) to represent the lung microenvironment in vivo. Our data demonstrate that PAV-104 inhibits SARS-CoV-2 production without affecting viral entry, mRNA transcription, or protein synthesis. PAV-104 interacts with SARS-CoV-2 nucleocapsid (N) and interferes with its oligomerization, blocking particle assembly. Transcriptomic analysis reveals that PAV-104 reverses SARS-CoV-2 induction of the type-I interferon response and the maturation of nucleoprotein signaling pathway known to support coronavirus replication. Our findings suggest that PAV-104 is a promising therapeutic candidate for COVID-19 with a mechanism of action that is distinct from existing clinical management approaches. PAV-104, a small molecule, potently inhibits SARS-CoV-2 replication in human airway epithelial cells by disrupting viral particle assembly and release. These findings suggest that PAV-104 holds significant promise as a therapeutic for COVID-19. Published (April 22, 2024): https://doi.org/10.1038/s42003-024-06130-8
|





 Your new post is loading...
Your new post is loading...