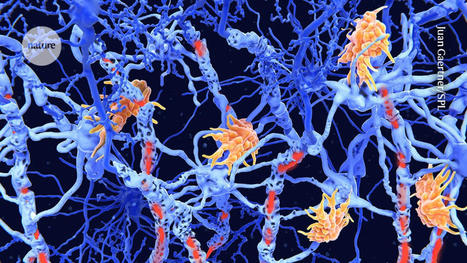
Studies in living brain tissue found that specialized immune cells in the brain can harbor latent but replication-competent HIV. As a part of its life cycle, human immunodeficiency virus-1 (HIV-1) inserts a copy of its DNA into human immune cells. Some of these newly infected immune cells can then transition into a dormant, latent state for a long period of time, which is known as HIV latency. Although current antiretroviral therapy (ART) against HIV can successfully block the virus from replicating further, it cannot eradicate latent HIV. If treatment is discontinued, the virus can rebound from latency and reignite the progression of HIV infection to AIDS. Scientists at the HIV Cure Center at the UNC School of Medicine, University of California, San Diego (UCSD), Emory University, and the University of Pennsylvania, have been searching for where exactly these latent cells are hiding in the body. Their newly reported studies indicate that brain microglial (BM)—specialized brain-resident immune cells with a decade-long lifespan—can serve as a stable viral reservoir for latent HIV. “We now know that microglial cells serve as a persistent brain reservoir,” said Yuyang Tang, PhD, assistant professor of medicine in the division of infectious diseases and member of the UNC HIV Cure Center. “This had been suspected in the past, but proof in humans was lacking. Our method for isolating viable brain cells provides a new framework for future studies on reservoirs of the central nervous system, and, ultimately, efforts towards the eradication of HIV.” Tang is first author of the team’s published paper in The Journal of Clinical Investigation, which is titled, “Brain microglia serve as persistent HIV reservoir despite durable antiretroviral therapy.”
HIV is a tricky virus to study. During infection, the virus specifically targets CD4+ lymphocytes which are the key coordinators of the immune response. Over time, the virus kills enough CD4+ cells to cause immunodeficiency. Past research has shown that latent HIV can hide within a few of the surviving CD4+ T cells throughout the body and the bloodstream. However, it’s been suspected that there are other viral reservoirs hidden within the central nervous system (CNS) in people with HIV who are receiving effective ART. But as the authors noted, “… rigorous evidence of viral persistence in the CNS cells of humans on durable suppressive ART is incomplete … Brain microglia (MG) may serve as a human immunodeficiency virus 1 (HIV) reservoir and ignite rebound viremia following cessation of antiretroviral therapy (ART), but they have yet to be proven to harbor replication-competent HIV. Unlike peripheral blood cells, it is extremely difficult to access and analyze brain tissues for the study of HIV reservoirs. Since these types of cells cannot be safely sampled in people taking ART, the potential viral reservoir in the brain has remained an enigma for many years. For their reported research the team first studied the brains of macaques infected with simian immunodeficiency virus (SIV), a virus that is closely related to HIV, from the Yerkes National Primate Research Center at Emory University to get a better understanding of how to extract and purify viable cells from primate brain tissue. The researchers used physical separation techniques and antibodies to selectively remove cells that were expressing microglial surface markers. Then, they isolated and separated the highly pure brain myeloid cells (BrMCs) from the CD4+ cells that were passing through the brain tissue. Using these techniques, researchers then obtained samples that were donated by HIV+ people (people with HIV; PWH) who were enrolled in “The Last Gift” Study at UCSD. As a part of this unique and important effort, altruistic HIV+ people, who are taking ART but suffering from other terminal illnesses, will their bodies to further the HIV research project.
“ … we first developed protocols to isolate highly pure populations of BrMCs and MG from the tissues of nonhuman primates (NHPs),” the authors explained. “We then adapted these protocols to the study of human brain tissues containing large numbers of viable cells after rapid autopsy to explore whether human BrMCs produce replication-competent HIV.” Co-author David Margolis, MD, the Sarah Kenan distinguished professor of medicine, microbiology & immunology, and epidemiology, further noted, “The samples are from people living with HIV, who are on therapy but facing a fatal disease of some kind. They were willing to not just donate their bodies to science, but also participate in the research program in the months leading up to their death. It’s an extraordinary program that made this critical research possible.” The scientists’ findings confirmed that MGs from an individual with HIV, being treated using ART, harbored replication-competent HIV. They acknowledged that although their study was limited by the small number of available samples from human donors on ART, they believe that the findings are consistent with NHP studies. “Our observations support the concept that brain MG are a stable reservoir of quiescent infection and may be a source of viral rebound upon treatment interruption,” they concluded. “Future efforts to clear HIV infection will have to include assessments of the persistence of HIV within CNS MG.” Now that the researchers know that latent HIV can take refuge in microglial cells in the brain, they are now considering plans to target this type of reservoir. Since latent HIV in the brain is radically different from the virus in the periphery, researchers believe that it has adapted special characteristics to replicate in the brain.
Reporting in their paper, the team noted, “Phenotyping studies characterized brain-derived virus as macrophage tropic based on the ability of the virus to infect cells expressing low levels of CD4. The lack of genetic diversity in virus from the brain suggests that this macrophage-tropic lineage quickly colonized brain regions.” NF-κB signaling is one of the critical signaling pathways that control HIV expression elsewhere in the body. When NF-κB signaling is “turned off,” HIV enters latency in the peripheral blood. However, latent HIV in the brain is not impacted by the activation of NF-κB signaling. Researchers are unsure why that is, but once an answer is found, they will be one step closer to knowing how to selectively target and eradicate the virus in the brain or peripheral blood. In addition to understanding the inner workings of the brain reservoir, the researchers are also trying to determine the true size of the latent HIV brain reservoir. “HIV is very smart,” said senior author Guochun Jiang, PhD, assistant professor in the UNC department of biochemistry and biophysics and member of the UNC HIV Cure Center. “Over time, it has evolved to have epigenetic control of its expression, silencing the virus to hide in the brain from immune clearance. We are starting to unravel the unique mechanism that allows latency of HIV in brain microglia”. Added Margolis, who is also the director of the UNC HIV Cure Center, “It is very hard to know how big the reservoir is. The problem with trying to eradicate HIV is like trying to eradicate cancer. You want to be able to get it all, so it won’t come back.”
Original research published in The Journal of Clin. Investigation (June 15, 2023):
https://doi.org/10.1172/JCI167417
Via Juan Lama





 Your new post is loading...
Your new post is loading...