Your new post is loading...
 Your new post is loading...
Authors: Lindy Abas, Martina Kolb, Johannes Stadlmann, Dorina P. Janacek, Kristina Lukic, Claus Schwechheimer, Leonid A. Sazanov, Lukas Mach, Jiří Friml, and Ulrich Z. Hammes.
PNAS (2021)
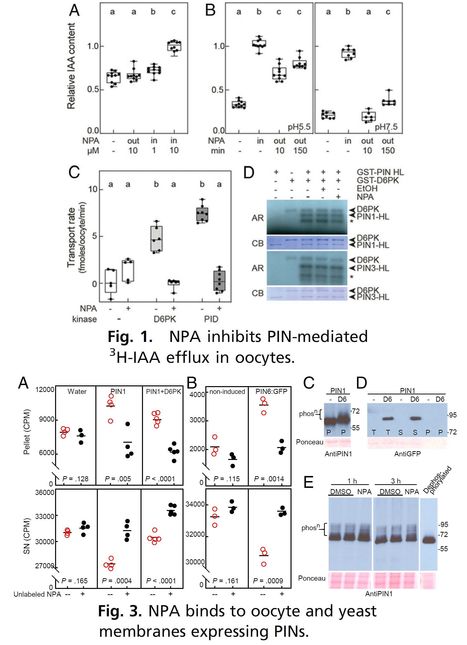
Significance: The plant hormone auxin regulates many aspects of plant life and has the unique ability to flow throughout the plant in defined directions, as observed by Darwin over a century ago. The chemical NPA inhibits this directional flow, thereby severely affecting plant growth. In studying the specific effects of NPA, researchers have also uncovered general principles underlying plant development. Exactly how NPA inhibits directional auxin flow is unclear. NPA interacts with proteins that can transport auxin, such as ABCB transporters, and we show here that NPA also associates with and inhibits the major transporters that specialize in directional auxin flow—PINs. This explanation of NPA action will guide future research and may help reveal how PINs perform auxin transport.
Abstract: "N-1-naphthylphthalamic acid (NPA) is a key inhibitor of directional (polar) transport of the hormone auxin in plants. For decades, it has been a pivotal tool in elucidating the unique polar auxin transport-based processes underlying plant growth and development. Its exact mode of action has long been sought after and is still being debated, with prevailing mechanistic schemes describing only indirect connections between NPA and the main transporters responsible for directional transport, namely PIN auxin exporters. Here we present data supporting a model in which NPA associates with PINs in a more direct manner than hitherto postulated. We show that NPA inhibits PIN activity in a heterologous oocyte system and that expression of NPA-sensitive PINs in plant, yeast, and oocyte membranes leads to specific saturable NPA binding. We thus propose that PINs are a bona fide NPA target. This offers a straightforward molecular basis for NPA inhibition of PIN-dependent auxin transport and a logical parsimonious explanation for the known physiological effects of NPA on plant growth, as well as an alternative hypothesis to interpret past and future results. We also introduce PIN dimerization and describe an effect of NPA on this, suggesting that NPA binding could be exploited to gain insights into structural aspects of PINs related to their transport mechanism."
Authors: Junzhe Wang, Xiaolong Guo, Qiang Xiao, Jianchu Zhu, Alice Y. Cheung, Li Yuan, Elizabeth Vierling and Shengbao Xu.
New Phytologist (2021)
Abstract: "The nucellus tissue in flowering plants provides nutrition for the development of the female gametophyte (FG) and young embryo. The nucellus degenerates as the FG develops, but the mechanism controlling the coupled process of nucellar degeneration and FG expansion remains largely unknown. The degeneration process of the nucellus and spatiotemporal auxin distribution in the developing ovule before fertilization were investigated in Arabidopsis thaliana. Nucellar degeneration before fertilization occurs through vacuolar cell death and in a ordered degeneration fashion. This sequential nucellar degeneration is controlled by the signalling molecule auxin. Auxin efflux plays the core role in precisely controlling the spatiotemporal pattern of auxin distribution in the nucellus surrounding the FG. The auxin efflux carrier PIN1 transports maternal auxin into the nucellus while PIN3/PIN4/PIN7 further delivers auxin to degenerating nucellar cells and concurrently controls FG central vacuole expansion. Notably, auxin level and auxin efflux are controlled by the maternal tissues, acting as a key communication from maternal to filial tissue."
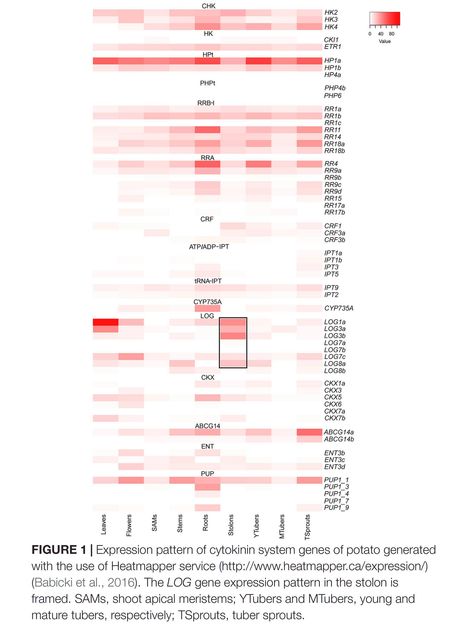
Authors: Sergey N. Lomin, Yulia A. Myakushina, Oksana O. Kolachevskaya, Irina A. Getman, Ekaterina M. Savelieva, Dmitry V. Arkhipov, Svetlana V. Deigraf and Georgy A. Romanov.
Frontiers in Plant Science (2020)
Abstract: "Cytokinins (CKs) were earlier shown to promote potato tuberization. Our study aimed to identify and characterize CK-related genes which constitute CK regulatory system in the core potato (Solanum tuberosum) genome. For that, CK-related genes were retrieved from the sequenced genome of the S. tuberosum doubled monoploid (DM) Phureja group, classified and compared with Arabidopsis orthologs. Analysis of selected gene expression was performed with a transcriptome database for the S. tuberosum heterozygous diploid line RH89-039-16. Genes responsible for CK signaling, biosynthesis, transport, and metabolism were categorized in an organ-specific fashion. According to this database, CK receptors StHK2/3 predominate in leaves and flowers, StHK4 in roots. Among phosphotransmitters, StHP1a expression largely predominates. Surprisingly, two pseudo-phosphotransmitters intended to suppress CK effects are hardly expressed in studied organs. Among B-type RR genes, StRR1b, StRR11, and StRR18a are actively expressed, with StRR1b expressing most uniformly in all organs and StRR11 exhibiting the highest expression in roots. By cluster analysis four types of prevailing CK-signaling chains were identified in (1) leaves and flowers, StHK2/3→StHP1a→StRR1b/+; (2) shoot apical meristems, stolons, and mature tubers, StHK2/4→StHP1a→StRR1b/+; (3) stems and young tubers, StHK2/4→StHP1a→StRR1b/11/18a; and (4) roots and tuber sprouts, StHK4→StHP1a→StRR11/18a. CK synthesis genes StIPT3/5 and StCYP735 are expressed mainly in roots followed by tuber sprouts, but rather weakly in stolons and tubers. By contrast, CK-activation genes StLOGs are active in stolons, and StLOG3b expression is even stolon-confined. Apparently, the main CK effects on tuber initiation are realized via activity of StLOG1/3a/3b/7c/8a genes in stolons. Current advances and future directions in potato research are discussed."
Authors: Hang Su, Tian Wang, Chuanfeng Ju, Jinping Deng, Tianqi Zhang, Mengjiao Li, Hui Tian and Cun Wang.
Journal of Integrative Plant Biology (2021)
Abstract: "Nitrogen (N) is a limiting nutrient for plant growth and productivity. The phytohormone abscisic acid (ABA) has been suggested to play a vital role in nitrate uptake in fluctuating N environments. However, the molecular mechanisms underlying the involvement of ABA in N deficiency responses are largely unknown. In this study, we demonstrated that ABA signaling components, particularly the three subclass III SUCROSE NON‐FERMENTING1 (SNF1)‐RELATED PROTEIN KINASE 2S (SnRK2) proteins, function in root foraging and uptake of nitrate under N deficiency in Arabidopsis thaliana. The snrk2.2snrk2.3snrk2.6 triple mutant grew a longer primary root and had a higher rate of nitrate influx and accumulation compared with wild‐type plants under nitrate deficiency. Strikingly, SnRK2.2/2.3/2.6 proteins interacted with and phosphorylated the nitrate transceptor NITRATE TRANSPORTER1.1 (NRT1.1) in vitro and in vivo. The phosphorylation of NRT1.1 by SnRK2s resulted in a significant decrease of nitrate uptake and impairment of root growth. Moreover, we identified NRT1.1Ser585 as a previously unknown functional site: the phosphomimetic NRT1.1S585D was impaired in both low‐ and high‐affinity transport activities. Taken together, our findings provide new insight into how plants fine‐tune growth via ABA signaling under N deficiency."
Authors: Zenglin Zhang, Mengmeng Xu and Yongfeng Guo.
Frontiers in Plant Science (2020)
Abstract: "Leaf senescence is regulated by a large number of internal and environmental factors. Here, we report that AtUSR1 (U-box Senescence Related 1) which encodes a plant Ring/U-box protein, is involved in age-dependent and dark-induced leaf senescence in Arabidopsis. Expression of AtUSR1 gene in leaves was up-regulated in darkness and during aging. Plants of usr1, an AtUSR1 gene knock-down mutant, showed a significant delay in age-dependent and dark-induced leaf senescence and the delayed senescence phenotype was rescued when the AtUSR1 gene was transferred back to the mutant plants. Meanwhile, overexpression of AtUSR1 caused accelerated leaf senescence. Furthermore, the role of AtUSR1 in regulating leaf senescence is related to MYC2-mediuated jasmonic acid (JA) signaling pathway. MeJA treatments promoted the accumulation of AtUSR1 transcripts and this expression activation was dependent on the function of MYC2, a key transcription factor in JA signaling. Dual-luciferase assay results indicated that MYC2 promoted the expression of AtUSR1. Overexpression of AtUSR1 in myc2 mutant plants showed precocious senescence, while myc2 mutation alone caused a delay in leaf senescence, suggesting that AtUSR1 functions downstream to MYC2 in the JA signaling pathway in promoting leaf senescence."
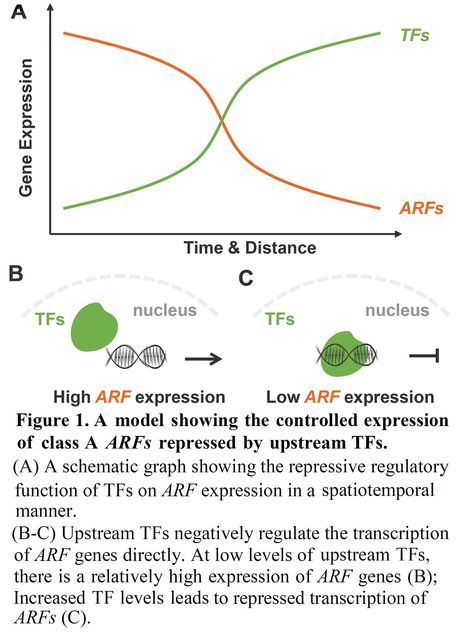
Author: Shutang Tan.
Molecular Plant (2021)
Excerpts: "The phytohormone auxin plays a pivotal role in shaping plant growth and development. It acts through a family of transcription factors called AUXIN RESPONSE FACTORs (ARFs) that regulate gene expression specifically. Five out of the 23 ARF transcription factors (TFs), also known as the class A, are transcription activators and key regulators in auxin-mediated plant development (Lavy and Estelle, 2016; Weijers and Wagner, 2016). Specific spatiotemporal expression pattern of the ARF genes enables asymmetric responsive capacity for distinct tissues. However, the underlying molecular mechanism remains largely unknown. A recent study reported that the tissue-specific expression patterns of the class A ARFs are realized through stringently-controlled transcriptions by upstream repressive TFs (Truskina et al., 2020)."
"In summary, the work by Truskina et al. (2020) identified a transcriptional network enabling the tissue-specific auxin signaling capacity through repressing distinct ARF expression by different combinations of TFs."
Authors: Henri Desaint, Nathalie Aoun, Laurent Deslandes, Fabienne Vailleau, Fabrice Roux and Richard Berthomé.
New Phytologist (2021)
Abstract: "In their natural environment, plants are exposed to biotic or abiotic stresses that occur sequentially or simultaneously. Plant responses to these stresses have been studied widely and have been well characterised in simplified systems involving single plant species facing individual stress. Temperature elevation is a major abiotic driver of climate change and scenarios have predicted an increase in the number and severity of epidemics. In this context, here we review the available data on the effect of heat stress on plant–pathogen interactions. Considering 45 studies performed on model or crop species, we discuss the possible implications of the optimum growth temperature of plant hosts and pathogens, mode of stress application and temperature variation on resistance modulations. Alarmingly, most identified resistances are altered under temperature elevation, regardless of the plant and pathogen species. Therefore, we have listed current knowledge on heat‐dependent plant immune mechanisms and pathogen thermosensory processes, mainly studied in animals and human pathogens, that could help to understand the outcome of plant–pathogen interactions under elevated temperatures. Based on a general overview of the mechanisms involved in plant responses to pathogens, and integrating multiple interactions with the biotic environment, we provide recommendations to optimise plant disease resistance under heat stress and to identify thermotolerant resistance mechanisms."
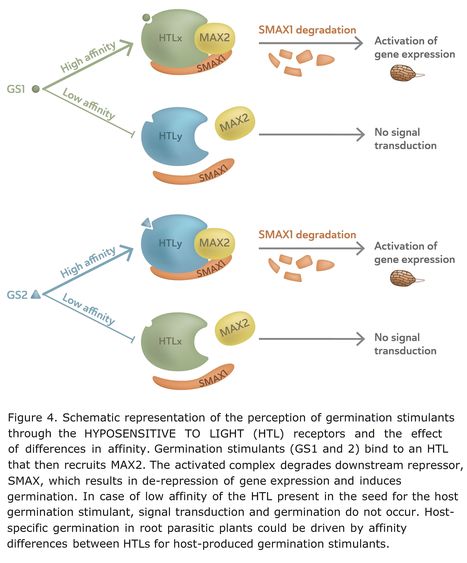
Authors: Harro Bouwmeester, Changsheng Li, Benjamin Thiombiano, Mehran Rahimi and Lemeng Dong.
Plant Physiology (2021)
Abstract: "Parasitic plants are plants that connect with a haustorium to the vasculature of another, host, plant from which they absorb water, assimilates, and nutrients. Because of this parasitic lifestyle, parasitic plants need to coordinate their lifecycle with that of their host. Parasitic plants have evolved a number of host detection/host response mechanisms of which the germination in response to chemical host signals in one of the major families of parasitic plants, the Orobanchaceae, is a striking example. In this Update Review, we discuss these germination stimulants. We review the different compound classes that function as germination stimulants, how they are produced, and in which host plants. We discuss why they are reliable signals, how parasitic plants have evolved mechanisms that detect and respond to them, and whether they play a role in host specificity. The advances in the knowledge underlying this signaling relationship between host and parasitic plant have greatly improved our understanding of the evolution of plant parasitism and are facilitating the development of more effective control measures in cases where these parasitic plants have developed into weeds."
Author: P. William Hughes.
The Plant Cell (2021)
Excerpts: "Although ethylene signalling is implicated in plant responses to heat stress, and ERF-mediated transcriptional regulation of downstream genes involved in thermo-tolerance is likely, the mechanism by which this is effected is currently unclear. New work by Huang et al., (2020) demonstrates that two ERFs (ERF95 and ERF97) are positive regulators of basal thermotolerance in Arabidopsis thaliana, thereby establishing that ethylene signalling in involved in heat stress response."
"In conclusion, the findings of Huang et al. (2020) establish that two ERFs, ERF95 and ERF97, are implicated in basal thermotolerance in Arabidopsis. Moreover, they also showed that many heat-response genes were found to be regulated by both ERFs in a heat-inducible manner. These findings significantly improve our understanding of how ethylene signalling may be involved in thermal tolerance."
Authors: John Patterson, Charles C. David, Marion Wood, Xiaolin Sun, Donald J. Jacobs and Erik H. A. Rikkerink.
bioRxiv (2020)
Non-expert Summary Statement: Gibberellins are plant hormones essential for growth and development. The DELLA proteins are a disordered family of repressors that transcriptionally repress GA responsive genes. Degradation of DELLA proteins in response to GA results in GA-responsive genes being upregulated. Binding of GA to the GA-Insensitive Dwarf 1 receptor (GID1) facilitates binding of DELLA to the GA ∙ GID1 complex. Through computational modelling and phylogenetic analyses, we identified a new GA binding cleft that is blocked by DELLA binding and a three-step mechanism for the GA ∙ DELLA ∙ GID1 complex that also involves the known GA binding channel. We propose a dual (cleft/channel) pathway that allows access to the binding pocket as a paradigm for selection of specific GA forms among a mixture of major active and inactive forms. The cleft is less selective, but preference for active GA in the binding pocket of GID1A is amplified by expunging inactive GA forms, followed by recruiting active forms through the more selective channel. This mechanism allows plants to sense concentration changes of GA with high specificity to enable certain GA variants to trigger specific signalling events. These novel insights into the receptor mechanism in part may explain the large number of different GA forms that exist in nature.
Abstract: "The hormone gibberellin (GA) promotes arabidopsis growth by enhancing binding between GA Insensitive DELLA transcriptional repressors and GA Insensitive Dwarf 1 (GID1) receptors to regulate DELLA degradation. The binding mechanism for GA was elucidated by employing a computational study of dissociations of the N-terminus of the DELLA family member GAI (GA Insensitive transcriptional repressor) from the GID1A receptor in the presence and absence of bound GA, and of GA from GID1A in the presence and absence of GAI. The tRAMD method was employed to deduce egression pathways for a diverse set of GA molecules (GA (x) ). Two pathways in the form of a newly identified cleft and a previously identified channel are prevalent. The cleft pathway is open in the absence of GAI. Upon GAI binding, the cleft route is blocked, resulting in a slower process for GA (x) to exit and enter the binding pocket through the channel. Several binding pocket residues are identified as gate-keepers to the channel. Molecular recognition features (MoRFs) found in the disordered signaling protein GAI affect GA (x) binding and GID1A dynamics. A three-step synergistic binding cycle is proposed where GAI MoRFs regulate the process. Rapid binding takes place through the cleft where little to no distinctions are made between major and less active forms of GA (x) . After GAI is bound to the GA (x) [[EQUATION]] GID1A complex, the channel supports a rectification process that increases the retention of major active forms of GA within the binding pocket. Both the cleft and channel contact residues to GA (x) are markedly conserved in a GID1 phylogeny, suggesting this binding process in the GID1 [[EQUATION]] DELLA GA-receptor complex represents a general paradigm for GA binding. Non-specific GA binding assists binding of GAI, which then helps to select the major active forms of the hormone and induce a downstream signalling cascade in response to bioactive GA."
Authors: Debabrata Panda and Jijnasa Barik.
Rice Science (2021)
Abstract: "Flooding is one of the most hazardous natural disasters and a major stress constraint to rice production throughout the world, which results in huge economic loss. Approximately one-fourth of the global rice crops (approximately 40 million hectares) are grown in rainfed lowland plots that are prone to seasonal flooding. A great progress has been made during last two decades in our understanding of the mechanisms involved in adaptation and tolerance to flooding/submergence in rice. In this review, we summarized the physiological and molecular mechanisms that contribute to tolerance of flooding/submergence in rice. We also covered various features of flooding stress with special reference to rice plants, viz. different types of flooding stress, environmental characterisation of flood water, impact of flooding stress on rice plant and their morphological, physiological and metabolic responses under flooding. A brief discussion on the tolerance mechanism in rice exhibited to different types of flooding will be focused for the future crop improvement programme for development of flooding tolerant rice variety."
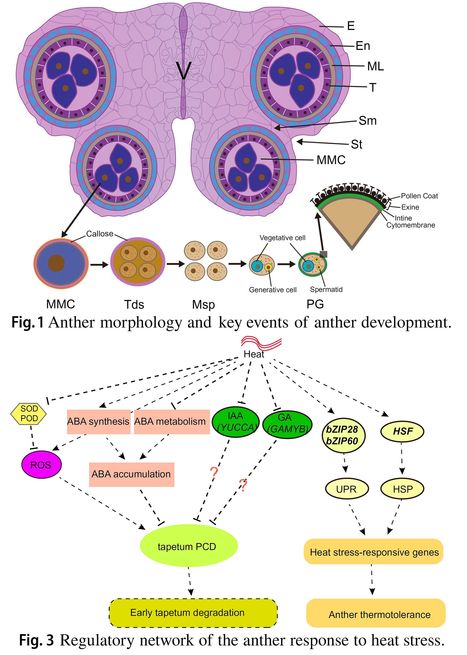
Authors: Zaibao Zhang, Menghui Hu, Weiwei Xu, Yuan Wang, Ke Huang, Chi Zhang and Jie Wen
Plant Molecular Biology (2021)
Key message: The developmental stage of anther development is generally more sensitive to abiotic stress than other stages of growth. Specific ROS levels, plant hormones and carbohydrate metabolism are disturbed in anthers subjected to abiotic stresses.
Abstract: "As sessile organisms, plants are often challenged to multiple extreme abiotic stresses, such as drought, heat, cold, salinity and metal stresses in the field, which reduce plant growth, productivity and yield. The development of reproductive stage is more susceptible to abiotic stresses than the vegetative stage. Anther, the male reproductive organ that generate pollen grains, is more sensitive to abiotic stresses than female organs. Abiotic stresses affect all the processes of anther development, including tapetum development and degradation, microsporogenesis and pollen development, anther dehiscence, and filament elongation. In addition, abiotic stresses significantly interrupt phytohormone, lipid and carbohydrate metabolism, alter reactive oxygen species (ROS) homeostasis in anthers, which are strongly responsible for the loss of pollen fertility. At present, the precise molecular mechanisms of anther development under adverse abiotic stresses are still not fully understood. Therefore, more emphasis should be given to understand molecular control of anther development during abiotic stresses to engineer crops with better crop yield."
Authors: Choonkyun Jung, Nguyen Hoai Nguyen and Jong-Joo Cheong.
International Journal of Molecular Sciences (2020)
Abstract: "The plant hormone abscisic acid (ABA) triggers cellular tolerance responses to osmotic stress caused by drought and salinity. ABA controls the turgor pressure of guard cells in the plant epidermis, leading to stomatal closure to minimize water loss. However, stomatal apertures open to uptake CO2 for photosynthesis even under stress conditions. ABA modulates its signaling pathway via negative feedback regulation to maintain plant homeostasis. In the nuclei of guard cells, the clade A type 2C protein phosphatases (PP2Cs) counteract SnRK2 kinases by physical interaction, and thereby inhibit activation of the transcription factors that mediate ABA-responsive gene expression. Under osmotic stress conditions, PP2Cs bind to soluble ABA receptors to capture ABA and release active SnRK2s. Thus, PP2Cs function as a switch at the center of the ABA signaling network. ABA induces the expression of genes encoding repressors or activators of PP2C gene transcription. These regulators mediate the conversion of PP2C chromatins from a repressive to an active state for gene transcription. The stress-induced chromatin remodeling states of ABA-responsive genes could be memorized and transmitted to plant progeny; i.e., transgenerational epigenetic inheritance. This review focuses on the mechanism by which PP2C gene transcription modulates ABA signaling."
|
Authors: Damiano Martignago, Beata Siemiatkowska, Alessandra Lombardi and Lucio Conti.
International Journal of Molecular Sciences (2020)
Abstract: "Plants can react to drought stress by anticipating flowering, an adaptive strategy for plant survival in dry climates known as drought escape (DE). In Arabidopsis, the study of DE brought to surface the involvement of abscisic acid (ABA) in controlling the floral transition. A central question concerns how and in what spatial context can ABA signals affect the floral network. In the leaf, ABA signaling affects flowering genes responsible for the production of the main florigen FLOWERING LOCUS T (FT). At the shoot apex, FD and FD-like transcription factors interact with FT and FT-like proteins to regulate ABA responses. This knowledge will help separate general and specific roles of ABA signaling with potential benefits to both biology and agriculture."
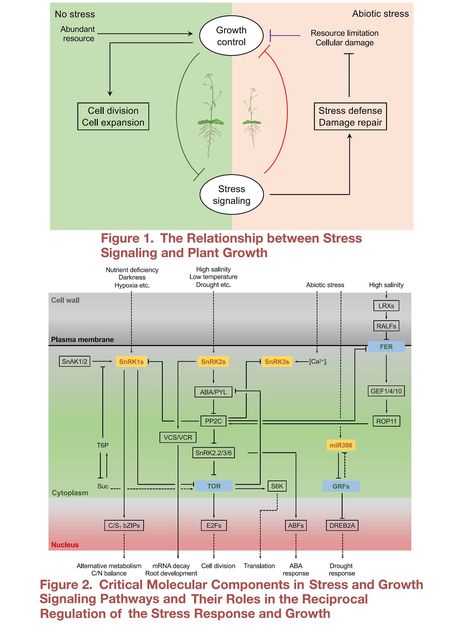
Authors: Heng Zhang, Yang Zhao and Jian-Kang Zhu.
Developmental Cell (2020)
Abstract: "Defense against stress and active suppression of growth are two complementary strategies by which plants respond to adverse environments. Although beneficial for plant survival, active growth inhibition is often undesirable for crop productivity. Compared with the knowledge on how plants defend against stress-caused cellular impairment, much less is known about how stress signaling regulates plant growth and vice versa. Here, we review recent progress in this area and discuss recent studies suggesting that reciprocal regulation between stress-response and growth-control pathways occurs at multiple levels. Understanding this regulatory network will be critical for resetting the balance between stress resistance and growth in order to engineer stress-resistant and high-yielding crops."
Authors: Bauyrzhan Smailov, Sanzhar Alybayev, Izat Smekenov, Aibek Mursalimov, Murat Saparbaev, Dos Sarbassov and Amangeldy Bissenbaev.
Frontiers in Cell and Developmental Biology (2020)
Abstract: "Germination is a process of seed sprouting that facilitates embryo growth. The breakdown of reserved starch in the endosperm into simple sugars is essential for seed germination and subsequent seedling growth. At the early stage of germination, gibberellic acid (GA) activates transcription factor GAMYB to promote de novo synthesis of isoforms of α-amylase in the aleurone layer and scutellar epithelium of the embryo. Here, we demonstrate that wheat germination is regulated by plant target of rapamycin (TOR) signaling. TOR is a central component of the essential-nutrient–dependent pathway controlling cell growth in all eukaryotes. It is known that rapamycin, a highly specific allosteric inhibitor of TOR, is effective in yeast and animal cells but ineffective in most of higher plants likely owing to structural differences in ubiquitous rapamycin receptor FKBP12. The action of rapamycin on wheat growth has not been studied. Our data show that rapamycin inhibits germination of wheat seeds and of their isolated embryos in a dose-dependent manner. The involvement of Triticum aestivum TOR (TaTOR) in wheat germination was consistent with the suppression of wheat embryo growth by specific inhibitors of the TOR kinase: pp242 or torin1. Rapamycin or torin1 interfered with GA function in germination because of a potent inhibitory effect on α-amylase and GAMYB gene expression. The TOR inhibitors selectively targeted the GA-dependent gene expression, whereas expression of the abscisic acid-dependent ABI5 gene was not affected by either rapamycin or torin1. To determine whether the TaTOR kinase activation takes place during wheat germination, we examined phosphorylation of a ribosomal protein, T. aestivum S6 kinase 1 (TaS6K1; a substrate of TOR). The phosphorylation of serine 467 (S467) in a hydrophobic motif on TaS6K1 was induced in a process of germination triggered by GA. Moreover, the germination-induced phosphorylation of TaS6K1 on S467 was dependent on TaTOR and was inhibited by rapamycin or torin1. Besides, a gibberellin biosynthesis inhibitor (paclobutrazol; PBZ) blocked not only α-amylase gene expression but also TaS6K1 phosphorylation in wheat embryos. Thus, a hormonal action of GA turns on the synthesis of α-amylase in wheat germination via activation of the TaTOR–S6K1 signaling pathway."
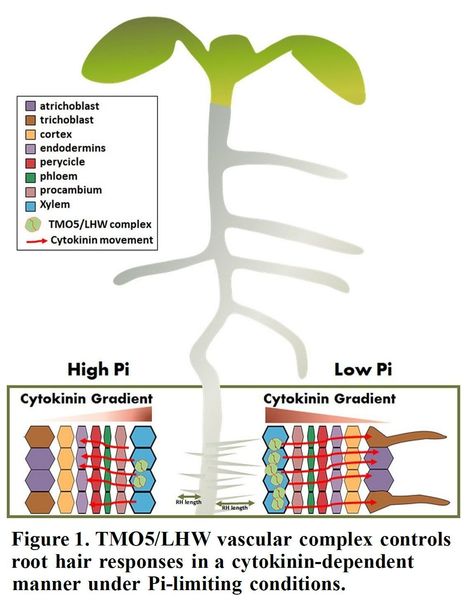
Author: Maida Romera-Branchat.
Molecular Plant (2021)
Excerpts: "Phosphorus (Pi) is a macronutrient essential for crucial plant biological processes including photosynthesis. To cope with the limited availability of Pi in the soil, plants have developed strategies such as increased hair root density to acquire this immobile nutrient. However, the precise molecular mechanisms involved in such strategies have not been fully elucidated. The production of bioactive cytokinin (CK) in the xylem was previously shown to be transcriptionally regulated by the bHLH transcription factors, TARGET OF MONOPTERS 5/LONESOME HIGHWAY (TMO5/LHW) dimeric complex. Although the movement of CK from the xylem to neighboring tissues such as the procambium is known to induce cell proliferation (De Rybel et al., 2014), how CK controls tissue growth beyond the vascular tissue was unknown."
"Altogether, this study indicates that the TMO5/LHW vascular complex functions cell non-autonomously to increase root hair density, and that CK is the mobile long-distance signal that depends on TMO5 to boost root hair growth under limiting Pi conditions (Figure 1). Understanding the genetic and molecular mechanisms of how plants respond to low Pi will guide the development of new cultivation strategies to avoid the overuse of fertilizers containing Pi."
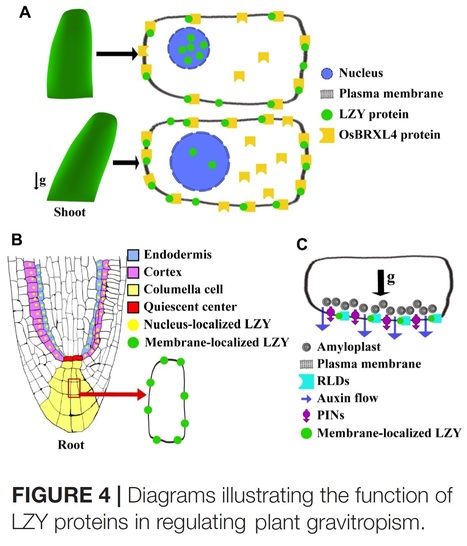
Authors: Zhicheng Jiao, Huan Du, Shu Chen, Wei Huang and Liangfa Ge.
Frontiers in Plant Science (2021)
Abstract: "Adapting to the omnipresent gravitational field was a fundamental basis driving the flourishing of terrestrial plants on the Earth. Plants have evolved a remarkable capability that not only allows them to live and develop within the Earth’s gravity field, but it also enables them to use the gravity vector to guide the growth of roots and shoots, in a process known as gravitropism. Triggered by gravistimulation, plant gravitropism is a highly complex, multistep process that requires many organelles and players to function in an intricate coordinated way. Although this process has been studied for several 100 years, much remains unclear, particularly the early events that trigger the relocation of the auxin efflux carrier PIN-FORMED (PIN) proteins, which presumably leads to the asymmetrical redistribution of auxin. In the past decade, the LAZY gene family has been identified as a crucial player that ensures the proper redistribution of auxin and a normal tropic response for both roots and shoots upon gravistimulation. LAZY proteins appear to be participating in the early steps of gravity signaling, as the mutation of LAZY genes consistently leads to altered auxin redistribution in multiple plant species. The identification and characterization of the LAZY gene family have significantly advanced our understanding of plant gravitropism, and opened new frontiers of investigation into the novel molecular details of the early events of gravitropism. Here we review current knowledge of the LAZY gene family and the mechanism modulated by LAZY proteins for controlling both roots and shoots gravitropism. We also discuss the evolutionary significance and conservation of the LAZY gene family in plants."
Authors: Xiaofeng Luo, Yujia Dai, Chuan Zheng, Yingzeng Yang, Wei Chen, Qichao Wang, Umashankar Chandrasekaran, Junbo Du, Weiguo Liu and Kai Shu.
New Phytologist (2021)
Abstract: "Salinity stress enhances reactive oxygen species (ROS) accumulation by activating the transcription of NADPH oxidase genes such as RbohD, thus mediating plant developmental processes, including seed germination. However, how salinity triggers the expression of ROS‐metabolism‐related genes and represses seed germination has not yet been fully addressed. In this study, we show that Abscisic Acid‐Insensitive 4 (ABI4), a key component in abscisic acid (ABA) signaling, directly combines with RbohD and Vitamin C Defective 2 (VTC2), the key genes involved in ROS production and scavenging, to modulate ROS metabolism during seed germination under salinity stress. Salinity‐induced ABI4 enhances RbohD expression by physically interacting with its promoter, and subsequently promotes ROS accumulation, thus resulting in cell membrane damage and a decrease in seed vigor. Additional genetic evidence indicated that the rbohd mutant largely rescues the salt‐hypersensitive phenotype of ABI4 overexpression seeds. Consistently, the abi4/vtc2 double mutant showed the salt‐sensitive phenotype, similar to the vtc2 mutant, suggesting that both RbohD and VTC2 are epistatic to ABI4 genetically. Altogether, these results suggest that the salt‐induced RbohD transcription and ROS accumulation is dependent on ABI4, and that the ABI4‐RbohD/VTC2 regulatory module integrates both ROS metabolism and cell membrane integrity, ultimately repressing seed germination under salinity stress."
Authors: Nathalie Kuhn, Claudio Ponce, Macarena Arellano, Alson Time, Boris Sagredo, José Manuel Donoso and Lee A. Meisel.
Plants (2020)
Abstract: "Several phytohormones modulate ripening in non-climacteric fruits, which is triggered by abscisic acid (ABA). Gibberellins (GAs) are present during the onset of ripening in sweet cherry fruits, and exogenous gibberellic acid (GA3) application delays ripening, though this effect is variety-dependent. Although an ABA accumulation delay has been reported following GA3 treatment, the mechanism by which GA modulates this process has not been investigated at the molecular level in sweet cherry. Therefore, the aim of this work is to analyze the effect of GA3 on the fruit ripening process and the transcript levels of ABA pathway orthologs in two varieties having different maturity time phenotypes. The early-season variety had a rapid transition from yellow to pink fruit color, whereas pink color initiation took longer in the mid-season variety. GA3 increased the proportion of lighter colored fruits at ripeness in both varieties, but it produced a delay in IAD—a ripening index—only in the mid-season variety. This delay was accompanied by an increased transcript abundance of PavPP2Cs, which are putative negative regulators of the ABA pathway. On the other hand, the early-season variety had increased expression of PavCYP707A2—a putative ABA catabolic gene–, and reduced transcript levels of PavPP2Cs and SnRK2s after the GA3 treatment. Together these results show that GA modulates fruit ripening, exerting its action in part by interacting with the ABA pathway in sweet cherry."
Authors: Arvid Herrmann and Keiko U. Torii.
Plant Physiology (2021)
Abstract: "Stomata are small pores on the surface of land plants that facilitate gas exchange for photosynthesis while minimizing water loss. The function of stomata is pivotal for plant growth and survival. Intensive research on the model plant Arabidopsis (Arabidopsis thaliana) has discovered key peptide signaling pathways, transcription factors, and polarity components that together drive proper stomatal development and patterning. In this review we focus on recent findings that have revealed co-option of peptide-receptor kinase signaling modules - utilized for diverse developmental processes and immune response. We further discuss an emerging connection between extrinsic signaling and intrinsic polarity modules. These findings have further enlightened our understanding of this fascinating developmental process."
Authors: Ruth Eichmann, Luke Richards and Patrick Schäfer.
The Plant Journal (2021)
Abstract: "The interaction of plants with complex microbial communities is the result of co‐evolution over millions of years and contributed to plant transition and adaptation to land. The ability of plants to be an essential part of complex and highly dynamic ecosystems is dependent on their interaction with diverse microbial communities. Plant microbiota can support, and even enable, the diverse functions of plants and are crucial in sustaining plant fitness under often rapidly changing environments. The composition and diversity of microbiota differs between plant and soil compartments. It indicates that microbial communities in these compartments are not static but are adjusted by the environment as well as inter‐microbial and plant‐microbe communication. Hormones take a crucial role in contributing to the assembly of plant microbiomes, and plants and microbes often employ the same hormones with completely different intentions. Here, the function of hormones as go‐betweens between plants and microbes to influence the shape of plant microbial communities is discussed. The versatility of plant and microbe‐derived hormones essentially contributes to the creation of habitats that are the origin of diversity and, thus, multi‐functionality of plants, their microbiota and ultimately ecosystems."
Authors: Jianyan Huang, Xiaobo Zhao, Marco Bürger, Yurong Wang and Joanne Chory.
The Plant Cell (2021)
Abstract: "The ETHYLENE RESPONSE FACTOR (ERF) transcription factors are integral components of environmental stress signaling cascades, regulating a wide variety of downstream genes related to stress responses and plant development. However, the mechanisms by which ERF genes regulate the heat stress response are not well understood. Here, we uncover the positive role of ethylene signaling, ERF95 and ERF97 in basal thermotolerance of Arabidopsis thaliana. We demonstrate that ethylene signaling-defective mutants exhibit compromised basal thermotolerance, whereas plants with constitutively activated ethylene response show enhanced basal thermotolerance. EIN3 physically binds to the promoters of ERF95 and ERF97. Ectopic constitutive expression of ERF95 or ERF97 increases the basal thermotolerance of plants. By contrast, erf95 erf96 erf97 erf98 quadruple mutants exhibit decreased basal thermotolerance. ERF95 and ERF97 genetically function downstream of EIN3. ERF95 can physically interact with ERF97, and this interaction is heat inducible. ERF95 and ERF97 regulate a common set of target genes, including known heat-responsive genes and directly bind to the promoter of HSFA2. Thus, our study reveals that the EIN3-ERF95/ERF97-HSFA2 transcriptional cascade may play an important role in the heat stress response, thereby establishing a connection between ethylene and its downstream regulation in basal thermotolerance of plants."
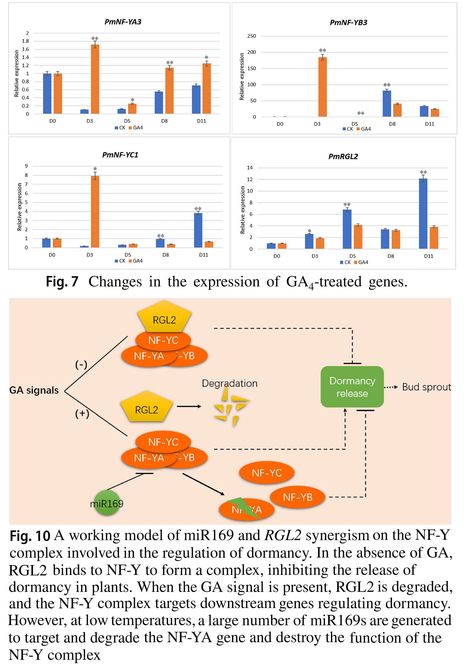
Authors: Jie Gao, Xiaopeng Ni, Hantao Li, Faisal Hayat, Ting Shi and Zhihong Gao.
Plant Molecular Biology (2021)
Key message: This study is the first to demonstrate that GA4-induced dormancy release is associated with the NF-Y complex, which interacts with gibberellin inhibitor RGL2 in Japanese apricot.
Abstract: "Seasonal dormancy is not only vital for the survival in cold winter but also affects flowering of temperate fruit trees and the dormancy release depends on the accumulation of the cold temperatures (Chilling requirement-CR). To understand the mechanism of dormancy release in deciduous fruit crops, we compared miRNA sequencing data during the transition stage from paradormancy to dormancy release in the Japanese apricot and found that the miR169 family showed significant differentially up-regulated expression during dormancy induction and was down-regulated during the dormancy release periods. The 5′ RACE assay and RT-qPCR validated its target gene NUCLEAR FACTOR-Y subunit A (NF-YA), which exhibited the opposite expression pattern. Further study showed that exogenous GA4 could inhibit the expression of the gibberellic acid (GA) signal transduction suppressor PmRGL2 (RGA-LIKE 2) and promote the expression of NF-Y. Moreover, the interaction between the NF-Y family and GA inhibitor PmRGL2 was verified by the yeast-two-hybrid (Y2H) system and a bimolecular fluorescence complementarity (BiFC) experiment. These results suggest that synergistic regulation of the NF-Y and PmRGL2 complex leads to the activation of dormancy release induced by GA4. These findings will help to elucidate the functional and regulatory roles of miR169 and NF-Y complex in seasonal bud dormancy induced by GA in Japanese apricot and provide new insights for the discovery of dormancy release mechanisms in woody plants."
Authors: Kiyohide Kojima, Daigo Andou and Masatake Ito.
The Horticulture Journal (2021)
Abstract: "To elucidate the phytohormone profiles associated with various stages of growing and ripening of small watermelon fruits, small watermelon fruits were collected 7 to 35 days after flowering (DAF) and divided into rind, pulp, and seeds. Indole-3-acetic acid (IAA), abscisic acid (ABA), trans-zeatin (tZ), isopentenyl adenine (iP), jasmonic acid (JA), methyl jasmonate (MeJA), gibberellin1 (GA1), and gibberellin4 (GA4) from each tissue were simultaneously quantified by liquid chromatography mass spectrometry (LC-MS). From 7 to 28 DAF, pulp weight increased rapidly due to cellular expansion. The IAA concentration increased up to 21 DAF in seeds, but then decreased up to 35 DAF. Similar changes occurred in the pulp approximately 7 days after the pattern of rising and falling IAA levels in the seeds. The GA concentration was higher in the seeds, and peaked prior to the seed growth peak. The ABA level in the pulp peaked at the time of fruit enlargement (21 DAF). The concentrations of iP, GA1, and GA4 tended to decrease with growth in all tissues. The results revealed the changes in endogenous plant hormones involved in the growth of small watermelon fruit."
|


 Your new post is loading...
Your new post is loading...