Your new post is loading...
 Your new post is loading...
Authors: Choonkyun Jung, Nguyen Hoai Nguyen and Jong-Joo Cheong.
International Journal of Molecular Sciences (2020)
Abstract: "The plant hormone abscisic acid (ABA) triggers cellular tolerance responses to osmotic stress caused by drought and salinity. ABA controls the turgor pressure of guard cells in the plant epidermis, leading to stomatal closure to minimize water loss. However, stomatal apertures open to uptake CO2 for photosynthesis even under stress conditions. ABA modulates its signaling pathway via negative feedback regulation to maintain plant homeostasis. In the nuclei of guard cells, the clade A type 2C protein phosphatases (PP2Cs) counteract SnRK2 kinases by physical interaction, and thereby inhibit activation of the transcription factors that mediate ABA-responsive gene expression. Under osmotic stress conditions, PP2Cs bind to soluble ABA receptors to capture ABA and release active SnRK2s. Thus, PP2Cs function as a switch at the center of the ABA signaling network. ABA induces the expression of genes encoding repressors or activators of PP2C gene transcription. These regulators mediate the conversion of PP2C chromatins from a repressive to an active state for gene transcription. The stress-induced chromatin remodeling states of ABA-responsive genes could be memorized and transmitted to plant progeny; i.e., transgenerational epigenetic inheritance. This review focuses on the mechanism by which PP2C gene transcription modulates ABA signaling."
Authors: Jian‐Ping An, Xiao‐Wei Zhang, Ya‐Jing Liu, Jiu‐Cheng Zhang, Xiao‐Fei Wang, Chun‐Xiang You and Yu‐Jin Hao.
The Plant Journal (2020)
Abstract: "Abscisic acid (ABA) induces chlorophyll degradation and leaf senescence. However, the molecular mechanism remains poorly understood, especially in woody plants. In this study, we found that MdABI5 plays an essential role in the regulation of ABA‐triggered leaf senescence in apple. Through yeast screening, three transcription factors, MdBBX22, MdWRKY40, and MdbZIP44, were found to interact directly with MdABI5 in vitro and in vivo. Physiological and biochemical assays showed that MdBBX22 delayed leaf senescence in two pathways. First, MdBBX22 interacted with MdABI5 to inhibit the transcriptional activity of MdABI5 on chlorophyll catabolic genes MdNYE1 and MdNYC1, thus negatively regulating chlorophyll degradation and leaf senescence. Second, MdBBX22 interacted with MdHY5 to interfere with the transcriptional activation of MdHY5 on MdABI5, thereby inhibiting the expression of MdABI5, which also contributed to the delay of leaf senescence. MdWRKY40 and MdbZIP44 were identified as positive regulators of leaf senescence. They accelerated MdABI5‐promoted leaf senescence through the same regulatory pathways, that is, interacting with MdABI5 to enhance the transcriptional activity of MdABI5 on MdNYE1 and MdNYC1. Taken together, our results suggest that MdABI5 works with its positive or negative interaction partners to regulate ABA‐mediated leaf senescence in apple, in which it acts as a core regulator. The antagonistic regulation pathways ensure that plants respond to external stresses flexibly and efficiently. Our results provide a concept for further study on the regulation mechanisms of leaf senescence."
Authors: Yuzhou Zhang, Lesia Rodriguez, Lanxin Li, Xixi Zhang and Jiří Friml.
Science Advances (2020)
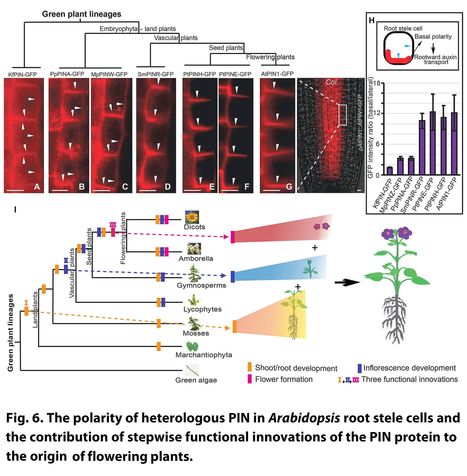
Abstract: "Flowering plants display the highest diversity among plant species and have notably shaped terrestrial landscapes. Nonetheless, the evolutionary origin of their unprecedented morphological complexity remains largely an enigma. Here, we show that the coevolution of cis-regulatory and coding regions of PIN-FORMED (PIN) auxin transporters confined their expression to certain cell types and directed their subcellular localization to particular cell sides, which together enabled dynamic auxin gradients across tissues critical to the complex architecture of flowering plants. Extensive intraspecies and interspecies genetic complementation experiments with PINs from green alga up to flowering plant lineages showed that PIN genes underwent three subsequent, critical evolutionary innovations and thus acquired a triple function to regulate the development of three essential components of the flowering plant Arabidopsis: shoot/root, inflorescence, and floral organ. Our work highlights the critical role of functional innovations within the PIN gene family as essential prerequisites for the origin of flowering plants."
Authors: Han-Qing Wang, Wei Xuan, Xin-Yuan Huang, Chuanzao Mao and Fang-Jie Zhao.
Plant and Cell Physiology (2020)
Abstract: "Cadmium (Cd) strongly inhibits root growth, especially the formation of lateral roots (LRs). The mechanism of Cd inhibition on LR formation in rice (Oryza sativa) remains unclear. In this study, we found that LR emergence in rice was inhibited significantly by 1 µM Cd and almost completely arrested by 5 µM Cd. Cadmium suppressed both the formation and subsequent development of the lateral root primordium (LRP). By using transgenic rice expressing the auxin response reporters DR5::GUS and DR5rev::VENUS, we found that Cd markedly reduced the auxin levels in the stele and LRP. Cadmium rapidly downregulated the expression of the auxin efflux transporter genes OsPIN1b, OsPIN1c and OsPIN9 in the stele and LPR. The emergence of LRs in a rice cultivar with a null allele of OsHMA3 (Heavy Metal ATPase 3) was more sensitive to Cd than cultivars with functional alleles. Overexpression of functional OsHMA3 in rice greatly alleviated the inhibitory effect of Cd, but the protective effect of OsHMA3 was abolished by the auxin polar transport inhibitor 1-N-naphthylphthalamic acid. The results suggest that Cd inhibits LR development in rice by disrupting OsPIN-mediated auxin distribution to LPR and OsHMA3 protects against Cd toxicity by sequestering Cd into the vacuoles."
Authors: Eric Brenya, Zhong-Hua Chen, David Tissue, Alexie Papanicolaou and Christopher Ian Cazzonelli.
BMC Plant Biology (2020)
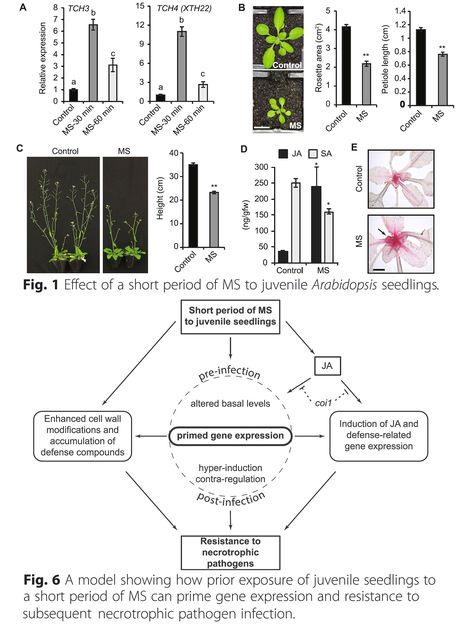
Abstract: "Prolonged mechanical stress (MS) causes thigmomorphogenesis, a stress acclimation response associated with increased disease resistance. What remains unclear is if; 1) plants pre-exposed to a short period of repetitive MS can prime defence responses upon subsequent challenge with necrotrophic pathogens, 2) MS mediates plant immunity via jasmonic acid (JA) signalling, and 3) a short period of repetitive MS can cause long-term changes in gene expression resembling a stress-induced memory. To address these points, 10-days old juvenile Arabidopsis seedlings were mechanically stressed for 7-days using a soft brush and subsequently challenged with the necrotrophic pathogens, Alternaria brassicicola, and Botrytis cinerea. Here we assessed how MS impacted structural cell wall appositions, disease symptoms and altered gene expression in response to infection. The MS-treated plants exhibited enhanced cell wall appositions and jasmonic acid (JA) accumulation that correlated with a reduction in disease progression compared to unstressed plants. The expression of genes involved in JA signalling, callose deposition, peroxidase and phytoalexin biosynthesis and reactive oxygen species detoxification were hyper-induced 4-days post-infection in MS-treated plants. The loss-of-function in JA signalling mediated by the JA-insensitive coronatine-insensitive 1 (coi1) mutant impaired the hyper-induction of defense gene expression and promoted pathogen proliferation in MS-treated plants subject to infection. The basal expression level of PATHOGENESIS-RELATED GENE 1 and PLANT DEFENSIN 1.2 defense marker genes were constitutively upregulated in rosette leaves for 5-days post-MS, as well as in naïve cauline leaves that differentiated from the inflorescence meristem well after ceasing MS. This study reveals that exposure of juvenile Arabidopsis plants to a short repetitive period of MS can alter gene expression and prime plant resistance upon subsequent challenge with necrotrophic pathogens via the JA-mediated COI1 signalling pathway. MS may facilitate a stress-induced memory to modulate the plant’s response to future stress encounters. These data advance our understanding of how MS primes plant immunity against necrotrophic pathogens and how that could be utilised in sustainable agricultural practices."
Authors: Akira Akamatsu, Miwa Nagae, Yuka Nishimura, Daniela Romero Montero, Satsuki Ninomiya, Mikiko Kojima, Yumiko Takebayashi, Hitoshi Sakakibara, Masayoshi Kawaguchi and Naoya Takeda.
The Plant Journal (2020)
Abstract: "Legumes and nitrogen‐fixing rhizobial bacteria establish root nodule symbiosis, which is orchestrated by several plant hormones. Exogenous addition of biologically active gibberellic acid (GA) is known to inhibit root nodule symbiosis. However, the precise role of GA has not been elucidated because of the trace amounts of these hormones in plants and the multiple functions of GAs. Here, we found that GA signaling acts as a key regulator in a long‐distance negative‐feedback system of root nodule symbiosis called autoregulation of nodulation (AON). GA biosynthesis is activated during nodule formation in and around the nodule vascular bundles, and bioactive GAs accumulate in the nodule. In addition, GA signaling induces expression of the symbiotic transcription factor NODULE INCEPTION (NIN) via a cis‐acting region on the NIN promoter. Mutants with deletions of this cis‐acting region have increased susceptibility to rhizobial infection and reduced GA‐induced CLE‐RS1 and CLE‐RS2 expression, suggesting that the inhibitory effect of GAs occurs through AON. This is supported by the GA‐insensitive phenotypes of an AON‐defective mutant of HYPERNODULATION ABERRANT ROOT FORMATION1 (HAR1), and a reciprocal grafting experiment. Thus, endogenous GAs induce NIN expression via its GA‐responsive cis‐acting region, then the GA‐induced NIN activates the AON system to regulate nodule formation."
Authors: Michael Bunsick and Shelley Lumba.
Plant Signaling & Behavior (2021)
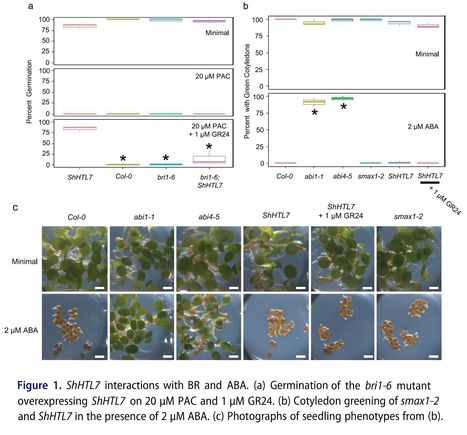
Abstract: "In the model plant Arabidopsis thaliana, two mutually antagonistic hormones regulate germination: abscisic acid (ABA) which promotes dormancy and gibberellins (GA) which breaks dormancy. Mutants auxotrophic for or insensitive to GA do not germinate. However, changes in the signaling flux through other hormone pathways will permit GA-independent germination. These changes include increased brassinosteroid (BR) signaling and decreased ABA signaling. Recently, strigolactone (SL) was also shown to enable GA-independent germination, provided the seeds express the SL receptor ShHTL7 from the parasitic plant Striga hermonthica. Here we show that a mutation which reduces sensitivity to BR (bri1-6) prevents ShHTL7 from promoting GA-independent germination. Further, we show that neither ShHTL7 nor the constitutive karrikin signaling mutant smax1-2 confer insensitivity to ABA. These results suggest ShHTL7 requires functional BR perception to bypass the GA requirement for germination."
Authors: Nana Dong, Wenchao Yin, Dapu Liu, Xiaoxing Zhang, Zhikun Yu, Wei Huang, Jihong Liu, Yanzhao Yang, Wenjing Meng, Mei Niu and Hongning Tong.
Frontiers in Plant Science (2020)
Abstract: "The complex roles of the steroid hormone brassinosteroids (BRs) in many different yield- and stress-related traits make it difficult to utilize the hormones for crop improvement. Here, we show that SERK2 as a BR signaling component is a potentially useful candidate for BR manipulation in rice. We generated multiple mutant alleles of SERK2 by CRISPR/Cas9 editing and show that knockout of SERK2 results in a compact structure accompanied with increased grain size. SERK2 is localized on plasma membrane and can interact with OsBRI1, the BR receptor, suggesting its conserved role as co-receptor in BR signaling. Consistently, the mutant has impaired BR sensitivity compared to wild type. Notably, the mutant is highly sensitive to salt stress as evaluated by plant survival rate as well as transcriptome analysis, whereas has slightly increased sensitivity to ABA, the stress hormone. By contrast, overexpression of SERK2 significantly enhances grain size and salt stress resistance, importantly, without affecting plant architecture. Furthermore, while salt suppresses SERK2 transcription, the protein is greatly induced by salt stress. Taken together, we propose that the adverse condition induces SERK2 accumulation to enhance early BR signaling on plasma membrane in favor of the anti-stress response. Our results illustrate the great potentials of specific BR components such as SERK2 for crop improvement by utilizing flexible strategies."
Authors: Yupu Huang, Sheliang Wang, Chuang Wang, Guangda Ding, Hongmei Cai, Lei Shi and Fangsen Xu.
Journal of Integrative Plant Biology (2020)
Abstract: "The essential micronutrient boron (B) has key roles in cell wall integrity and B deficiency inhibits plant growth. The role of jasmonic acid (JA) in plant growth inhibition under B deficiency remains unclear. Here, we report that low B elevates JA biosynthesis in Arabidopsis thaliana by inducing the expression of JA biosynthesis genes. Treatment with JA inhibited plant growth and, a JA biosynthesis inhibitor enhanced plant growth, indicating that the JA induced by B deficiency affects plant growth. Furthermore, examination of the JA signaling mutants jasmonate resistant1, coronatine insensitive1‐2, and myc2 showed that JA signaling negatively regulates plant growth under B deficiency. We identified a low‐B responsive transcription factor, ERF018, and used yeast one‐hybrid assays and transient activation assays in Nicotiana benthamiana leaf cells to demonstrate that ERF018 activates the expression of JA biosynthesis genes. ERF018 overexpression (OE) lines displayed stunted growth and up‐regulation of JA biosynthesis genes under normal B conditions, compared to Col‐0 and the difference between ERF018 OE lines and Col‐0 diminished under low B. These results suggest that ERF018 enhances JA biosynthesis and thus negatively regulates plant growth. Taken together, our results highlight the importance of JA in the effect of low B on plant growth."
Authors: Niels Aerts, Marciel Pereira Mendes and Saskia C.M. Van Wees.
The Plant Journal (2021)
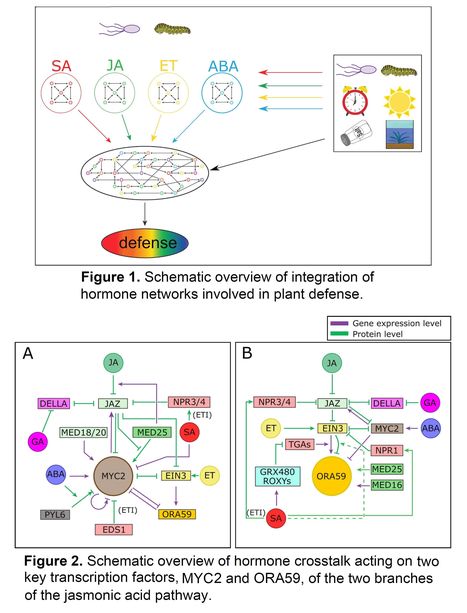
Abstract: "Plant hormones are essential for regulating the interactions between plants and their complex biotic and abiotic environments. Each hormone initiates a specific molecular pathway and these different hormone pathways are integrated in a complex network of synergistic, antagonistic and additive interactions. The inter‐pathway communication is called hormone crosstalk. By influencing the immune network topology, hormone crosstalk is essential for tailoring plant responses to diverse microbes and insects in diverse environmental and internal contexts. Crosstalk provides robustness to the immune system but also drives specificity of induced defense responses against the plethora of biotic interactors. Recent advances in dry‐lab and wet‐lab techniques have greatly enhanced our understanding of the broad‐scale effects of hormone crosstalk on immune network functioning and have revealed underlying principles of crosstalk mechanisms. Molecular studies have demonstrated that hormone crosstalk is modulated at multiple levels of regulation, such as by affecting protein stability, gene transcription and hormone homeostasis. These new insights into hormone crosstalk regulation of plant defense are reviewed here, with a focus on crosstalk acting on the jasmonic acid pathway in Arabidopsis thaliana, pinpointing to the transcription factors MYC2 and ORA59 as major targets for modulation by other hormones."
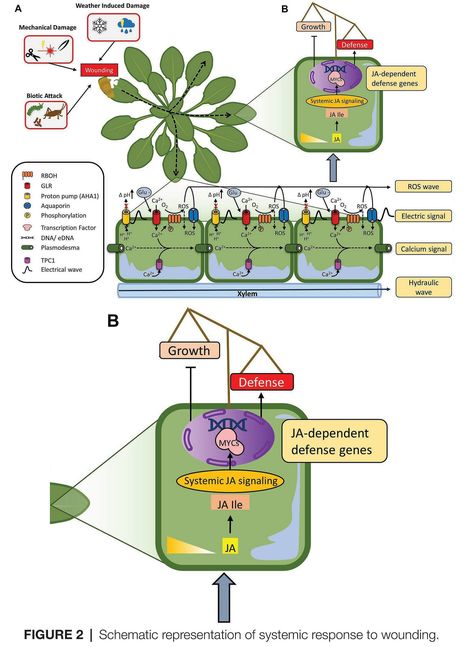
Authors: Isaac Vega-Muñoz, Dalia Duran-Flores, Álvaro Daniel Fernández-Fernández, Jefri Heyman, Andrés Ritter and Simon Stael.
Frontiers in Plant Science (2020)
Abstract: "Recognition and repair of damaged tissue are an integral part of life. The failure of cells and tissues to appropriately respond to damage can lead to severe dysfunction and disease. Therefore, it is essential that we understand the molecular pathways of wound recognition and response. In this review, we aim to provide a broad overview of the molecular mechanisms underlying the fate of damaged cells and damage recognition in plants. Damaged cells release the so-called damage associated molecular patterns to warn the surrounding tissue. Local signaling through calcium (Ca2+), reactive oxygen species (ROS), and hormones, such as jasmonic acid, activates defense gene expression and local reinforcement of cell walls to seal off the wound and prevent evaporation and pathogen colonization. Depending on the severity of damage, Ca2+, ROS, and electrical signals can also spread throughout the plant to elicit a systemic defense response. Special emphasis is placed on the spatiotemporal dimension in order to obtain a mechanistic understanding of wound signaling in plants."
Authors: Yuliya A. Krasylenko, George Komis, Sonya Hlynska, Tereza Vavrdová, Miroslav Ovečka, Tomáš Pospíšil and Jozef Šamaj.
bioRxiv (2020)
Abstract: "Strigolactones are phytohormones involved in shoot branching and hypocotyl elongation. The latter phenomenon was addressed herein by the exogenous application of a synthetic strigolactone GR24 and an inhibitor of strigolactone biosynthesis TIS108 on hypocotyls of wild type Arabidopsis and a strigolactone signalling mutant max2-1 (more axillary growth 2-1). Owing to the interdependence between light and strigolactone signalling, the present work was extended to seedling cultivation under a standard light/dark regime, or under continuous darkness. Given the essential role of the cortical microtubules in cell elongation, their organization and dynamics were characterized under the conditions of altered strigolactone signalling using fluorescence microscopy methods with different spatiotemporal capacities such as confocal laser scanning microscopy and structured illumination microscopy. It was found that the strigolactone-dependent inhibition of hypocotyl elongation correlated with changes in cortical microtubule organization and dynamics, visualized in living wild type and max2-1 seedlings stably expressing genetically-encoded fluorescent molecular markers for microtubules. Quantitative analysis of microscopic datasets revealed that chemical and/or genetic manipulation of strigolactone signalling affected microtubule remodelling, especially under light conditions. The application of GR24 and TIS108 in dark conditions partially alleviated cytoskeletal rearrangement, suggesting a new mechanistic connection between the cytoskeletal behaviour and the light-dependence of strigolactone signalling."
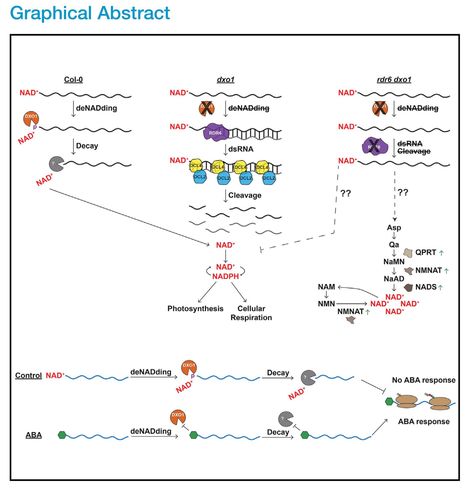
Authors: Xiang Yu, Matthew R. Willmann, Lee E. Vandivier, Sophie Trefely, Marianne C. Kramer, Jeffrey Shapiro, Rong Guo, Eric Lyons, Nathaniel W. Snyder and Brian D. Gregory.
Developmental Cell (2020)
Editor's view: Eukaryotic RNAs have been found to contain 50-end NAD+ caps, but the dynamic nature of these modifications is unknown. Yu et al. demonstrate that plant response to the phytohormone abscisic acid (ABA) reshapes RNA NAD+ capping to stabilize transcripts encoding ABA response proteins, allowing proper response to this essential phytohormone.
Highlights: • Messenger RNA NAD+ capping is widespread and varies between tissues in plants • NAD+-capped RNAs are unstable and a lack of their turnover induces NAD+ metabolism • Plant response to abscisic acid (ABA) remodels the NAD+-capped transcriptome • Much of the ABA-mediated NAD+ transcriptome reprogramming does not require DXO1
Abstract: "Although eukaryotic messenger RNAs (mRNAs) normally possess a 5′ end N7-methyl guanosine (m7G) cap, a non-canonical 5′ nicotinamide adenine dinucleotide (NAD+) cap can tag certain transcripts for degradation mediated by the NAD+ decapping enzyme DXO1. Despite this importance, whether NAD+ capping dynamically responds to specific stimuli to regulate eukaryotic transcriptomes remains unknown. Here, we reveal a link between NAD+ capping and tissue- and hormone response-specific mRNA stability. In the absence of DXO1 function, transcripts displaying a high proportion of NAD+ capping are instead processed into RNA-dependent RNA polymerase 6-dependent small RNAs, resulting in their continued turnover likely to free the NAD+ molecules. Additionally, the NAD+-capped transcriptome is significantly remodeled in response to the essential plant hormone abscisic acid in a mechanism that is primarily independent of DXO1. Overall, our findings reveal a previously uncharacterized and essential role of NAD+ capping in dynamically regulating transcript stability during specific physiological responses."
|
Authors: Alon Israeli, Yogev Burko, Sharona Shleizer-Burko, Iris Daphne Zelnik, Noa Sela, Mohammad R. Hajirezaei, Alisdair R Fernie, Takayuki Tohge, Naomi Ori and Maya Bar.
bioRxiv (2020)
Abstract: "Morphogenesis and differentiation are important stages in organ development and shape determination. However, how they are balanced and tuned during development is not fully understood. In the compound leaved tomato, an extended morphogenesis phase allows for the initiation of leaflets, resulting in the compound form. Maintaining a prolonged morphogenetic phase in early stages of compound-leaf development is dependent on delayed activity of several factors that promote differentiation, including CIN-TCP transcription factor (TF) LA, the MYB TF CLAU and the plant hormone Gibberellin (GA). Here, we investigated the genetic regulation of the morphogenesis-differentiation balance by studying the relationship between LA, CLAU and GA. Our genetic and molecular examination suggest that LA is expressed more broadly than CLAU and determines the spatio-temporal context of CLAU activity. We demonstrate that both LA and CLAU affect the Cytokinin/Gibberellin (CK/GA) balance. LA reduces the sensitivity of the leaf margin to CK, shown before to be also affected by CLAU. CLAU affects leaf active GA content and sensitivity, shown previously to be also influenced by LA. Therefore, LA and CLAU likely function in parallel pathways to promote leaf differentiation by converging on common downstream processes, including the CK/GA balance."
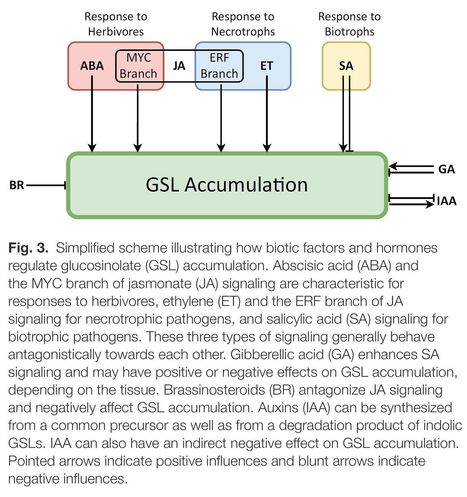
Author: Simon Mitreiter and Tamara Gigolashvili.
Journal of Experimental Botany (2020)
Abstract: "Glucosinolates are secondary defense metabolites produced by plants of the order Brassicales, which includes the model species Arabidopsis and many crop species. In the past 13 years, the regulation of glucosinolate synthesis in plants has been intensively studied, with recent research revealing complex molecular mechanisms that connect glucosinolate production with responses to other central pathways. In this review, we discuss how the regulation of glucosinolate biosynthesis is ecologically relevant for plants, how it is controlled by transcription factors, and how this transcriptional machinery interacts with hormonal, environmental, and epigenetic mechanisms. We present the central players in glucosinolate regulation, MYB and basic helix–loop–helix transcription factors, as well as the plant hormone jasmonate, which together with other hormones and environmental signals allow the coordinated and rapid regulation of glucosinolate genes. Furthermore, we highlight the regulatory connections between glucosinolates, auxin, and sulfur metabolism and discuss emerging insights and open questions on the regulation of glucosinolate biosynthesis."
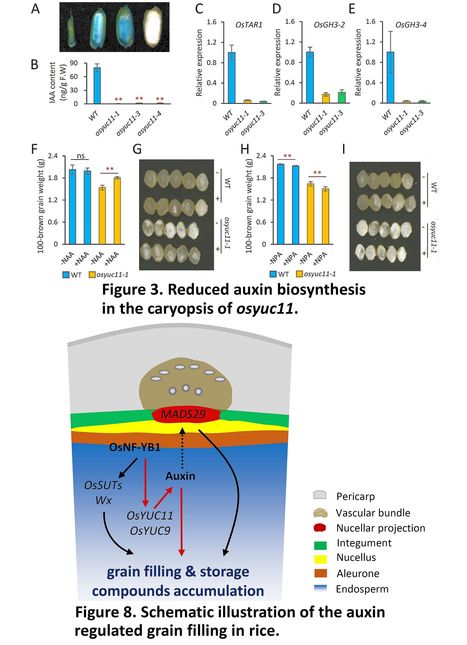
Authors: Xinyu Xu, Zhiguo E, Dongping Zhang, Qianbin Yun, Yong Zhou, Baixiao Niu and Chen Chen.
Plant Physiology (2021)
Abstract: "Auxin is a phytohormone essential for plant development. However, our understanding of auxin-regulated endosperm development remains limited. Here, we described rice YUCCA (YUC) flavin-containing monooxygenase encoding gene OsYUC11 as a key contributor to auxin biosynthesis in rice (Oryza sativa) endosperm. Grain filling or storage product accumulation was halted by mutation of OsYUC11, but the deficiencies could be recovered by exogenous application of auxin. A rice transcription factor (TF) yeast library was screened, and 41 TFs that potentially bind to the OsYUC11 promoter were identified, of which OsNF-YB1, a member of the nuclear factor Y family, is predominantly expressed in the endosperm. Both osyuc11 and osnf-yb1 mutants exhibited reduced seed size and increased chalkiness, accompanied by a reduction in indole-3-acetic acid biosynthesis. OsNF-YB1 can bind the OsYUC11 promoter to induce gene expression in vivo. We also found that OsYUC11 was a dynamically imprinted gene that predominantly expressed the paternal allele in the endosperm up to 10 days after fertilization (DAF) but then became a non-imprinted gene at 15 DAF. A functional maternal allele of OsYUC11 was able to recover the paternal defects of this gene. Overall, the findings indicate that OsYUC11-mediated auxin biosynthesis is essential for endosperm development in rice."
Authors: Graham M. Burkart and Federica Brandizzi.
Trends in Biochemical Sciences (2021)
Highlights: Plant and mammalian target of rapamycin (TOR) signaling pathways share many conserved methods of activation, inhibition, and effectors, and regulate some overlapping cellular processes; however, some of the mammalian TOR core components are missing in plants. TOR signaling in plants has evolved to regulate multiple plant-specific processes, some using known TOR effectors from mammalian TOR signaling. Crosstalk between TOR and phytohormone signaling enables plants to modulate the growth response to adapt to a variety of biotic and abiotic stressors.
Abstract: "To identify the appropriate times for growth and development, organisms must sense and process information about the availability of nutrients, energy status, and environmental cues. For sessile eukaryotes such as plants, integrating such information can be critical in life or death decisions. For nearly 30 years, the conserved phosphatidylinositol 3-kinase-related protein kinases (PIKKs) target of rapamycin (TOR) has been established as a central hub for integrating external and internal metabolic cues. Despite the functional conservation across eukaryotes, the TOR complex has evolved specific functional and mechanistic features in plants. Here, we present recent findings on the plant TOR complex that highlight the conserved and unique nature of this critical growth regulator and its role in multiple aspects of plant life."
Authors: Faiza Ali, Zhenzhen Wei, Yonghui Li, Lei Gan, Zuoren Yang, Fuguang Li and Zhi Wang.
bioRxiv (2020)
Abstract: "Seed vigor is an important trait for ecology, agronomy, and economy and varies with different plant species and environmental conditions. Dehydration-Responsive Element-Binding Protein 2B (DREB2B), a subgroup of the DREB transcription factor family, is well-known in drought resistance. However, the role of DREB2B in the regulation of seed vigor has not been identified. Here, we found that DREB2B is a negative regulator of seed vigor by ABA-mediated pathway in Arabidopsis with loss of function mutant and over-expressed transgenic lines. Furthermore, DREB2B showed epistatic and parallel to ABI3 simultaneously in seed vigor regulation by genetic and molecular approaches. DREB2B homolog gene (GhDREB2B-A09) was also identified in cotton. The expression analysis indicated that transcripts of DREB2B were higher in mature dry seed, and the transgenic plants showed the conservative roles of DREB2B in Arabidopsis and cotton. In addition, we identified that DREB2B interacted with RADICAL-INDUCED CELL DEATH1 (RCD1) to involve seed vigor regulation together in Arabidopsis and cotton with BiFC experiment and mutant phenotypic analysis. Collectively it is concluded that DREB2B interacting with RCD1 or SRO1 function at upstream of and synergistic with ABI3 to regulate seed vigor negatively in Arabidopsis and cotton, which provides novel knowledge in the seed development study."
Authors: Kai Chen, Weican Liu, Xiaowei Li and Haiyan Li.
Plant Signaling & Behavior (2021)
Abstract: "GmGASA is the GASA gibberellin regulated cysteine-rich protein family. The expression of GmGASA is up-regulated by gibberellin, which is the longest plant hormone in plants playing vital roles in plant development. However, very few reports explaining the direct regulation of downstream genes by GASA gene are available. In the current study, the GmGASA32, a member of the GASA family affecting soybean height was identified. In the early stage, preliminary verification of the response of GmGASA32 to gibberellin through phenotypic experiment was done. The promoter activity analysis confirmed that GmGASA32 was induced by gibberellin. Subcellular localization showed that GmGASA32-GFP fusion protein enriched in the nucleus after gibberellin treatment. In order to confirm the function of GmGASA32 in the nucleus, we confirmed that the GASA domain in the C terminal of GmGASA32 can interact with GmCDC25 (cell cycle-associated protein) through the bimolecular fluorescence complementation (BiFC) assay."
Authors: Aatifa Rasool, Sheikh Mansoor, K. M. Bhat, G. I. Hassan, Tawseef Rehman Baba, Mohammed Nasser Alyemeni, Abdulaziz Abdullah Alsahli, Hamed A. El-Serehy, Bilal Ahmad Paray and Parvaiz Ahmad.
Frontiers in Plant Science (2020)
Abstract: "Grafting is a common practice for vegetative propagation and trait improvement in horticultural plants. A general prerequisite for successful grafting and long term survival of grafted plants is taxonomic proximity between the root stock and scion. For the success of a grafting operation, rootstock and scion should essentially be closely related. Interaction between the rootstock and scion involves complex physiological-biochemical and molecular mechanisms. Successful graft union formation involves a series of steps viz., lining up of vascular cambium, generation of a wound healing response, callus bridge formation, followed by vascular cambium formation and subsequent formation of the secondary xylem and phloem. For grafted trees compatibility between the rootstock/scion is the most essential factor for their better performance and longevity. Graft incompatibility occurs on account of a number of factors including of unfavorable physiological responses across the graft union, transmission of virus or phytoplasma and anatomical deformities of vascular tissue at the graft junction. In order to avoid the incompatibility problems, it is important to predict the same at an early stage. Phytohormones, especially auxins regulate key events in graft union formation between the rootstock and scion, while others function to facilitate the signaling pathways. Transport of macro as well as micro molecules across long distances results in phenotypic variation shown by grafted plants, therefore grafting can be used to determine the pattern and rate of recurrence of this transport. A better understanding of rootstock scion interactions, endogenous growth substances, soil or climatic factors needs to be studied, which would facilitate efficient selection and use of rootstocks in the future. Protein, hormones, mRNA and small RNA transport across the junction is currently emerging as an important mechanism which controls the stock/scion communication and simultaneously may play a crucial role in understanding the physiology of grafting more precisely. This review provides an understanding of the physiological, biochemical and molecular basis underlying grafting with special reference to horticultural plants."
Authors: Ahmed Khadr, Guang-Long Wang, Ya-Hui Wang, Rong-Rong Zhang, Xin-Rui Wang, Zhi-Sheng Xu, Yong-Sheng Tian and Ai-Sheng Xiong.
Peer J (2020)
Abstract: "Carrot is an important root vegetable crop abundant in bioactive compounds including carotenoids, vitamins, and dietary fibers. Carrot intake and its products are gradually growing owing to its high antioxidant activity. Auxins are a class of plant hormones that control many processes of plant growth and development. Yet, the effects of exogenous application of auxin on lignin biosynthesis and gene expression profiles of lignin-related genes in carrot taproot are still unclear. In order to investigate the effect of exogenous indole-3-butyric acid (IBA) on lignin-related gene profiles, lignin accumulation, anatomical structures and morphological characteristics in carrot taproots, carrots were treated with different concentrations of IBA (0, 50, 100, and 150 µM). The results showed that IBA application significantly improved the growth parameters of carrot. The 100 or 150 µM IBA treatment increased the number and area of xylem vessels, whereas transcript levels of lignin-related genes were restricted, resulting in a decline in lignin content in carrot taproots. The results indicate that taproot development and lignin accumulation may be influenced by the auxin levels within carrot plants.
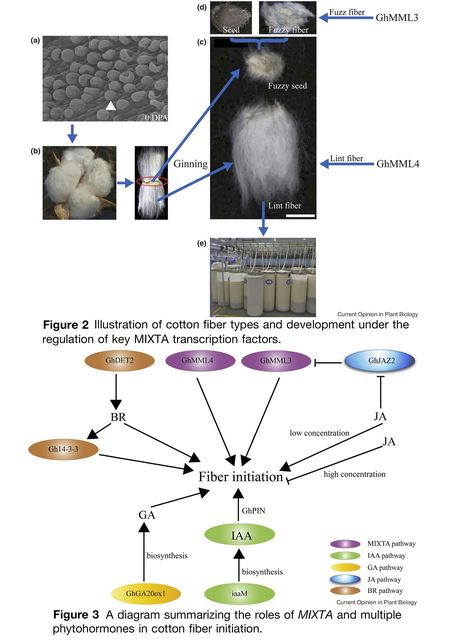
Authors: Yue Tian and Tianzhen Zhang.
Current Opinion in Plant Biology (2021)
Highlights: • Seed trichome or fiber in cotton serves as an ideal model to study plant cell differentiation. • Fiber initiation determines cotton fiber yield. • GhMMLs evolve important and unique roles in cotton fiber initiation. • GhMMLs and phyhormones act together to regulate cotton fiber development.
Abstract: "Cotton is the largest source of natural fiber for textile industry in the world. Cotton fibers are seed trichomes that make cotton unique among plants. Cotton fibers originate from ovule epidermal cells and serve as an excellent model to study the process of cell differentiation in plants. Characterization of factors contributing to fiber development will help to reveal general mechanisms of cell differentiation in plants. Transcription factors (TFs), especially MYB-MIXTA-like (MML) factors, appear to have evolved unique roles in fiber development. In addition, phytohormones including brassinosteroids, jasmonic acid, GA and auxin also play an important role in regulating fiber development. Here, we summarize the mechanisms of MIXTAs and phytohormones orchestrating cotton fiber development. The progress in understanding molecular basis of fiber development will facilitate future genetic engineering and breeding to improve cotton fiber quality and yield."
Authors: Jun Takeuchi, Kosuke Fukui, Yoshiya Seto, Yousuke Takaoka and Masanori Okamoto.
The Plant Journal (2021)
Abstract: "Small‐molecule plant hormones principally control plant growth, development, differentiation, and environmental responses. Nine types of plant hormones are ubiquitous in angiosperms, and the molecular mechanisms of their hormone actions have been elucidated during the last two decades by genomic decoding of model plants with genetic mutants. In particular, the discovery of hormone receptors has greatly contributed to the understanding of signal transduction systems. The three‐dimensional structure of the ligand–receptor complex has been determined for eight of the nine hormones by X‐ray crystal structure analysis, and ligand perception mechanisms have been revealed at the atomic level. Collective research has revealed the molecular function of plant hormones that act as either molecular glue or an allosteric regulator for activation of receptors. In this review, we introduce an overview of the respective hormone signal transduction and describe the structural bases of ligand–receptor interactions."
Authors: Kinuka Ohtaka, Akiko Yoshida, Yusuke Kakei, Kosuke Fukui, Mikiko Kojima, Yumiko Takebayashi, Kanako Yano, Shunsuke Imanishi and Hitoshi Sakakibara.
Frontiers in Plant science (2020)
Abstract: "Temperature is a critical environmental factor governing plant growth and development. The difference between day temperature (DT) and night temperature (NT), abbreviated as DIF, influences plant architecture. Subjecting plants to artificial DIF treatments is an effective strategy in ornamental horticulture. For example, negative DIF (when DT – NT Solanum lycopersicum) seedlings grown under different DIF treatments. Under positive DIF (when DT – NT > 0), in contrast to the control temperature (25°C/20°C, DT/NT), high temperature (30°C/25°C) increased stem length and thickness, as well as the number of xylem vessels. Conversely, compared with the positive high temperature DIF treatment (30°C/25°C), under negative DIF treatment (25°C/30°C) stem elongation was inhibited, but stem thickness and the number of xylem vessels were not affected. The negative DIF treatment decreased the expression of gibberellin (GA)-, auxin-, and cell wall-related genes in the epicotyl, as well as the concentrations of GAs and indole-3-acetic acid (IAA). The expression of these genes and concentrations of these hormones increased under high temperature compared to those under the control temperature positive DIF. Our results suggest that stem length in tomato seedlings is controlled by changes in GA and IAA biosynthesis in response to varying day and night temperatures."
Authors: Likai Wang, Zhiyuan Zhang, Fan Zhang, Zhengyao Shao, Bo Zhao, Austin Huang, Jaclyn Tran, Fernando Vera Hernandez and Hong Qiao.
The Plant Cell (2021)
Abstract: "Ethylene is an important phytohormone with pleotropic roles in plant growth, development, and stress responses. ETHYLENE INSENSITIVE2 (EIN2) mediates the transduction of the ethylene signal from the endoplasmic reticulum membrane to the nucleus, where its C-terminus (EIN2-C) regulates histone acetylation to mediate transcriptional regulation by EIN3. However, no direct interaction between EIN2-C and EIN3 has been detected. To determine how EIN2-C and EIN3 act together, we followed a synthetic approach and engineered a chimeric EIN2-C with EIN3 DNA binding activity but lacking its transactivation activity (EIN2C-EIN3DB). Overexpression of EIN2C-EIN3DB in either wild type or in the ethylene insensitive mutant ein3-1 eil1-1 led to a partial constitutive ethylene response. ChIP-seq showed that EIN2C-EIN3DB has DNA binding activity, indicating that EIN3DB is functional in EIN2C-EIN3DB. Furthermore, native EIN3 protein levels determine EIN2C-EIN3DB binding activity and binding targets in a positive feedback loop by interacting with EIN2C-EIN3DB to form a heterodimer. Additionally, although EIN3 does not direct affect histone acetylation levels in the absence of EIN2, it is required for the ethylene-induced elevation of H3K14Ac and H3K23Ac in the presence of EIN2. Together, we reveal efficient and specific DNA binding by dimerized EIN3 in the presence of ethylene to mediate positive feedback regulation, which is required for EIN2-directed elevation of histone acetylation to integrate into an EIN3-dependent transcriptional activation."
|


 Your new post is loading...
Your new post is loading...